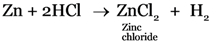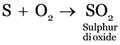Materials: Metals and non-metals Worksheet-5
-
Non-metals can not be drawn into wires. Why?
-
Complete the following equation :
Zn + HCl → ___ + ___
-
Give two examples of noble metals.
-
Give the name of one non-metal which is a good conductor of electricity.
-
Which is the most abundant metal on the earth's crust?
-
Which metal can be extracted from magnetite?
-
Name the ore from which aluminium is extracted.
-
Why is aluminium used for making cooking utensils?
-
Which of the following can be beaten into thin sheets?
(a) Zinc (b) Phosphorus
(c) Sulphur (d) Oxygen.
-
Name the metals that occur in free state.
-
Name the non-metal that occurs in free state.
-
Metals are (softer/harder) than non-metals.
-
Most non-metals are (bad/good) conductors of heat.
-
The property that allows the metals to be hammered into thin sheets is called (ductility/malleability).
-
Melting point of most non-metals is (higher/lower) than metals.
-
(Metals/non-metals) display lustre.
-
The product/s formed on dissolution of magnesium oxide in water is/are _____.
-
The product/s formed on burning sulphur in air is ____.
-
The product/s formed on reaction between sodium and water is/are ____.
-
The product/s formed on reaction between Aluminium and hydrochloric acid is/are _____.
Answer:
-
Non metals exist as gas, liquid or soft solids. They are not ductile hence they cannot be drawn into wires.
-
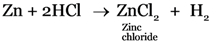
-
The noble metals are metals that are resistant to corrosion and oxidation in moist air, unlike most base metals. Few examples are Palladium, Silver, Platinum, Gold.
-
Graphite is allotropic form of carbon which is good conductor of electricity.
(Explanation: The arrangement of carbon atoms in graphite is such that one electron of each carbon atom remains free which is responsible for electrical conductivity).
-
Aluminium is the most abundant metal on Earth’s crust.
-
Iron can be extracted from Magnetite. It is the ore of iron and consists of oxides of iron.
-
Aluminium is extracted from Bauxite. Its chemical formula is Al2O3.2H2O
-
Aluminium is good conductor of heat. It is also a cheap and light metal hence it is used for making cooking utensils.
-
Zinc can be beaten into sheets as it is a metal.
-
Metals like gold, silver, platinum, are noble metals. They occur in free state.
-
Helium occurs in free state.
-
Metals are harder than non-metals. Diamond an (allotrope of carbon) is an exception. It is a hard non-metal.
-
Most non-metals are bad conductors of heat.
-
Malleability
-
Melting point of most non-metals is lower than metals.
-
Metals are lustrous.
-
The product formed is Magnesium hydroxide.
Chemical equation:
MgO + H2O → Mg(OH)2
-
The product formed is Sulphur dioxide.
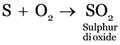
-
Sodium hydroxide and hydrogen gas
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
-
Aluminium chloride and hydrogen gas
2Al + 6HCl → 2AlCl3 + 2H2