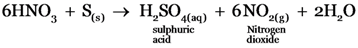Materials: Metals and non-metals Worksheet-6
-
The product/s formed on reaction between sulphur and hot concentrated nitric acid is /are_______
-
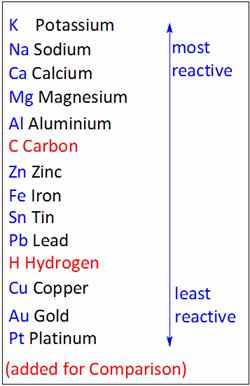
Arrange the following metals in the order of their decreasing chemical activity: magnesium, potassium, iron, gold.
-
Can copper displace iron from iron sulphate solution? Give reasons.
-
(Platinum/iron) is the member of-the family of noble metals.
-
Gold dissolves in (aqua regia/aqueous solution of silver nitrate).
-
Silver tarnishes due to (nitrogen oxides/hydrogen sulphide) in the air.
-
Why is tincture iodine used to protect wounds from germs ?
-
Explain the use of chlorine in water purification plants.
-
Why is aluminium used in making aeroplanes?
-
What types of oxides are formed by metals?
-
What types of oxides are formed by non-metals?
-
How does phosphorus occur in nature?
-
Which metal foil is used in packing of some medicine tablets?
-
Name the soft metal which can be cut with a knife.
-
Name the non-metal used in vulcanization.
-
Name one metal which is not malleable.
-
Name one non-metal which has lustre.
-
Name one non-metal which is a good conductor of electricity.
-
What would happen to iron railings if they are not painted?
-
Name the element commonly used for converting edible vegetable oils into vanaspati ghee.
-
Name the metal whose salt is used for making photographic films.
-
A set of metals in order of their increasing chemical reactivity is given below:
silver, copper, lead, iron, zinc, magnesium and sodium.
Which metals will react with cold water?
-
A set of metals in order of their increasing chemical reactivity is given below:
silver, copper, lead, iron, zinc, magnesium and sodium.
Which gas will be liberated when metals react with cold water?
-
A set of metals in order of their increasing chemical reactivity is given below:
silver, copper, lead, iron, zinc, magnesium and sodium.
Which of the metals will react with oxygen when heated?
-
A set of metals in order of their increasing chemical reactivity is given below:
silver, copper, lead, iron, zinc, magnesium and sodium.
Which of the metals become black in the presence of hydrogen sulphide?
Answer:
-
Sulphuric acid, nitrogen dioxide and water.
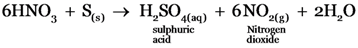
-
The reactivity of given metals is in the order:
Potassium>Magnesium> Iron> Gold
-
Copper cannot displace iron from iron sulphate solution because copper is less reactive than iron. Iron is placed above copper in the metal reactivity series hence is more reactive than copper.
-
Platinum is a noble metal. It doesn’t get corroded.
-
Gold dissolves in aqua regia. Aqua regia is 3 parts HCl and 1 part HNO3.
-
Silver tarnishes in air due to Hydrogen sulphide. Silver reacts with Hydrogen sulphide forming Silver sulphide.
2Ag + H2S → Ag2S + H2
-
Tincture iodine is an antiseptic hence it is used in wounds. Tincture iodine contains 2 to 7 % elemental iodine along with sodium and potassium iodide dissolved in ethanol and water.
-
Chlorine is a germicide and disinfectant. Chlorine is an oxidizing agent hence used as germicide in water purification process.
-
Alloy of aliminium- duralumin (95% Al, 4% Cu, 0.5% Mg, 0.5% Mn) its strength is comparable to mild steels hence used for making aeroplanes.
-
Metals form basic oxides.
-
Non metals form acidic and neutral oxides.
-
Phosphorous occurs in nature as minerals. Some of the minerals of phosphorous are Rock phosphate or phosphorite (Ca3(PO4)2) , Fluoroaptite, Chloroaptite.
-
Aluminium foil is used in packing of medicine tablets.
-
Sodium, potassium are some of the soft metals that can be cut with knife.
-
Sulphur is used in vulcanization of rubber.
-
Sodium is one of the metals which is not malleable. It is a soft metal and can be cut with knife.
-
Diamond an allotropic form of carbon has lustre.
-
Graphite an allotrope of carbon is good conductor of electricity.
-
A reddish brown coloured substance called rust will be deposited on the iron railings if they are not painted.
-
Hydrogen is used for converting edible oils into vanaspati ghee.
-
Salt of silver i.e. silver bromide is used for making photographic films.
-
Sodium
-
Hydrogen gas is liberated when metals react with water.
-
Magnesium will react with oxygen when heated.
-
Silver will become black in the presence of Hydrogen sulphide due to formation of Silver sulphide.
2Ag + H2S → Ag2S + H2(g)