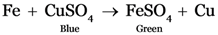Materials: Metals and non-metals Worksheet-4
A. Red B. Blue C. Green D. Yellow
A. Sodium B. Potassium
C. Carbon D. Phosphorous
A. Aluminium B. Silver
C. Nickeloy D. Magnelium
A. Copper and Tin B. Copper and Zinc
C. Copper and Nickel D. Copper and Iron
A. Cu(OH)2 and CuCO3 B. Cu(OH)2 and CuO
C. CuCO3 and CuO D. CuO and Cu2O
A. Aluminium sulphate B. Copper sulphate
C. Sodium sulphate D. Magnesium sulphate
A. Acidic is nature B. Neutral
C. Basic in nature D. Either acidic or basic
A. Chlorine B. Iodine C. Oxygen D. Sulphur
A. less B. more
A. Rust is basic iron oxide
B. Rust is a mixture of FeO and Fe2(CO3)3
C. Rust is hydrated Ferric oxide.
D. Rust is a mixture of FeO, Fe2(CO3)3Fe(OH)3
A. metals B. Semi metals
C. Non-metal D. All of these
A. Acid B. Base
C. Salt D. Both acid & base
A. Zinc B. Iron C. Aluminium D. Sulphur
A. H2O+CO2+O2 B. H2O+CO+O2
C. H2O+CO2+H2 D. H2O+O2+H2
A. The colour of the solution does not change
B. The colour of the solution change from blue to green
C. The colour of the solution change from green to blue
D. The solution becomes colourless
Answer:
Explanation: Thin foils of aluminium are used in wrapping soaps, cigarettes and confectionary.
Explanation: Bronze used for making coins, utensils, statues is an alloy of copper and tin.
(Cu-75-90%, Sn 25 -10%)
Explanation: The green material is a mixture of copper hydroxide (Cu(OH)2) and copper carbonate
(CuCO3).
Explanation: Zinc will replace copper from copper sulphate forming Zinc sulphate.
Zn + CuSO4 → ZnSO4 + Cu
The blue colour of copper sulphate disappears when zinc granules are added to copper sulphate solution. Powdery red mass of copper is deposited at the bottom of the vessel.
Aluminium, sodium and magnesium are more reactive than zinc so these metals will replace zinc from its salt solution.
Explanation: Aqueous solution of metallic oxides is alkaline in nature. It changes red litmus blue.
Explanation: Chlorine is used for purification of water.
Explanation: In the metal reactivity series iron is placed below zinc.
Explanation: Iron metal reacts with air and moisture forming hydrated iron oxide.
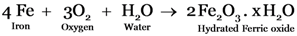
Explanation: Example:
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
2Al + 6HCl → 2AlCl3 + 3H2
Explanation: Sulphur is a non metal hence it can’t be beaten into sheets.
Explanation: Iron replaces copper from copper sulphate forming iron sulphate. Thus the colour of the solution changes from blue to green. Reddish brown deposits of copper can be seen at the bottom of test tube.