SEPARATION OF CONSTITUENTS FROM HOMOGENEOUS MIXTURES
-
In a homogeneous mixture the composition is uniform and components of mixture are present in single phase.
-
It is either a mixture of some miscible liquids or a liquid in which some dissolved salts are present as impurities (e.g. sea water).
Methods of separation:
Separation of non volatile and volatile substance form mixture
Distillation:
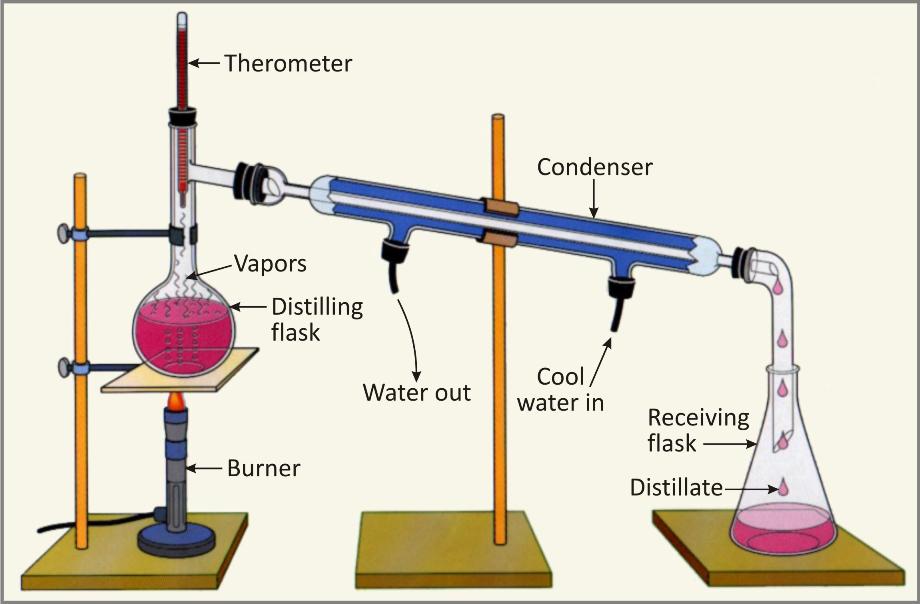
Process:
-
The impure solution is taken in a glass flask known as distillation flask. It is provided with a thermometer to record the temperature and an outlet for the vapours.
-
Water will change into vapours once it starts boiling. These vapours escaping from the flask are passed through a condenser.
-
Water is kept circulating around it. Vapours will get condensed into liquid form which is completely pure. It can be collected in the receiver as shown in the flask.
-
The impurities being non-volatile in nature will be left behind in the flask.
Example:
Purification of sea water
-
Sea water contains dissolved salts (e.g., sodium chloride, potassium iodide etc.) as impurities. These salts are non-volatile in nature i.e. these salts do not change into vapours even on strong heating.
-
The purification of sea water can be done with the help of a technique called distillation or simple distillation.
Separation of constituents from a Miscible Liquid Mixture:
-
Separation is based upon the difference in the boiling points of the constituents present.
Constituents differing in boiling point by more than 25°C (Separation of acetone and water)
-
The boiling point temperature of acetone is 56°C (319 K) while that of water is 100°C (373 K). The separation can be done in the distillation flask.
Process:
-
Take the liquid mixture in the distillation flask and heat from below. Since the boiling point temperature of acetone (56°C) is less than that of water, it will change into vapours which will rise upwards.
-
They will get condensed on passing through the condenser and collect in the receiver. At this temperature, water will not boil and will remain in the flask.
-
Thus, the process of distillation helps in separating acetone and water.
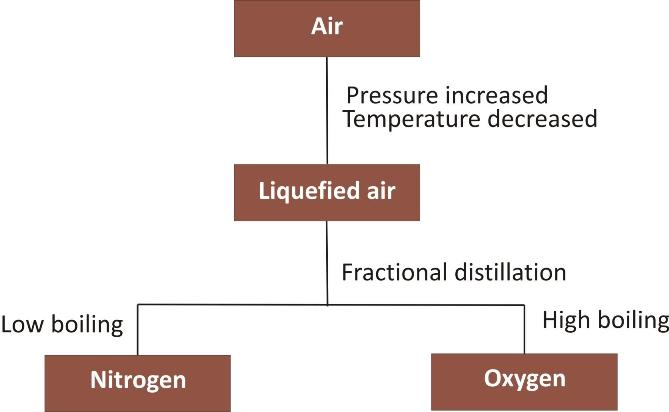
Separation of gases from air
-
Air is a homogeneous mixture of a number of gases like nitrogen, oxygen, inert gases and carbon dioxide etc.
-
These can be separated from air by fractional distillation.
-
In fact, air is first liquefied by increasing the pressure and decreasing the temperature.
-
The liquefied air is then subjected to fractional distillation when the gas with lesser boiling point gets distilled first.
-
For example, the boiling points of the main gases nitrogen (78.1% by volume) and oxygen (20.9% by volume) are -196°C and -182°C respectively. When the liquefied air is distilled fractionally, nitrogen will be distilled first and oxygen afterwards.
Flow chart for the separation of nitrogen and oxygen from air
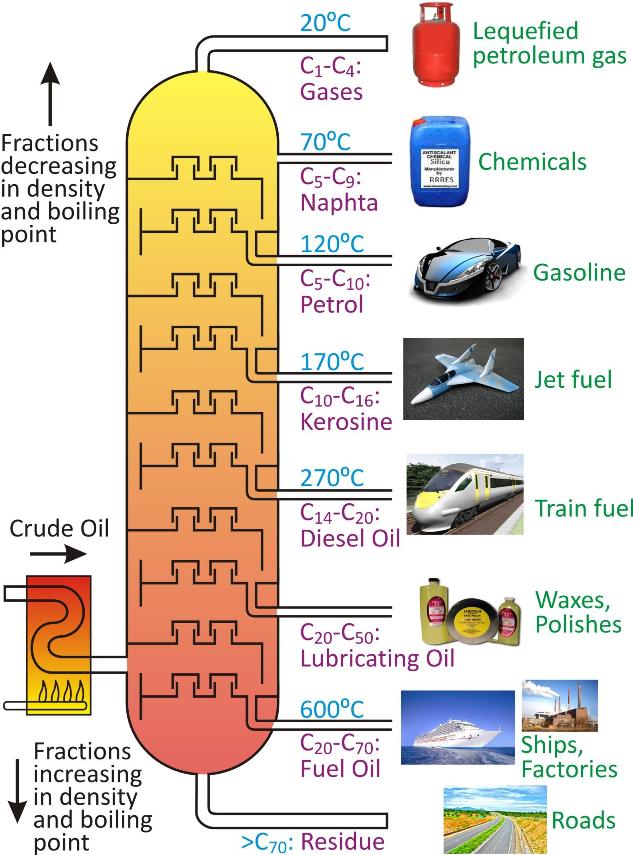
Fractional distillation of crude oil
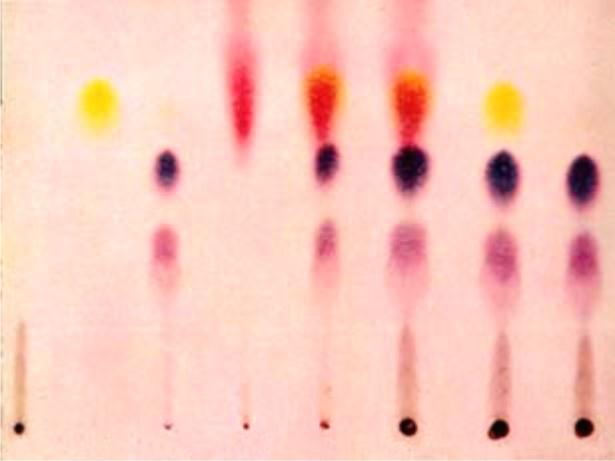
Chromatography for the separation of constituents from a mixture:
-
In Greek, the word ‘Kroma’ means colour and the word chromatography implies ‘writing in colour’. Actually, this technique was initially used to separate coloured components from pigments and dyes.
-
Chromatography helps in separating and identifying the components present in homogeneous mixtures which may be available even in very small amount.
Process:
-
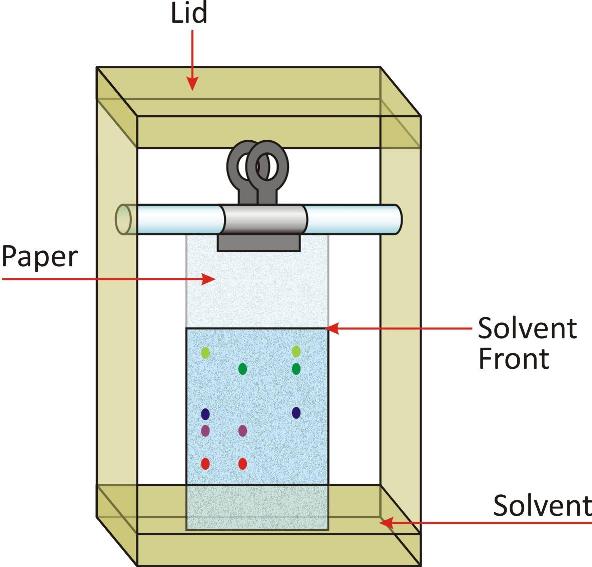
Paper chromatography is very simple and is commonly used in the laboratory.
-
With the help of this technique we can identify the blue as well black components in a drop of the ink which is water soluble.
-
In order to perform the experiment, a strip of very fine filter paper (25 x 5 cm) is taken.
-
A fine line is drawn on the paper from one end approximately at a distance of 3 cm.
-
A small drop of blue/ black ink mixture is put in the centre of the line with the help of a fine capillary tube.
-
Now the strip is suspended in a jar containing a small amount of water so that the end of strip which has been marked dips in water to a length of about 0-5 to 1 cm.
-
The strip is allowed to stand undisturbed in the jar for about 20 to 30 minutes.
-
Due to capillary action, water will rise upwards.
-
Blue/black ink is actually a mixture of blue and black dyes. Both the components present in the mixture will rise upwards.
-
The more soluble will move faster.
-
Paper chromatography helps in separating and identifying the components present in homogeneous mixtures which may be available even in very small amount.
-
It also helps in checking the purity of a given sample. It may not be possible to discuss more details at this level of the students.
Question:
How will you separate the constituents present in the following mixtures.
-
Common salt and water
-
Iodine and sand
-
Kerosene and water
-
Sugar and sulphur.
Solution:
-
Common salt and water:
-
Since common salt (sodium chloride) is soluble in water, it can be separated by crystallisation. The process of distillation can also be used because sodium chloride is non-volatile and water is volatile in nature.
-
Iodine and sand:
-
Sublimation process can be used. Iodine will sublime on heating while sand will remain unaffected.
-
Kerosene and water:
-
The liquids are not miscible with each other. Separation can be done by using a separating funnel.
-
Sugar and sulphur:
-
The mixture is dissolved in carbon disulphide in a beaker by stirring with a glass rod. Sulphur dissolves while sugar remains as such. On filtering, sugar separates as the residue. The filtrate upon concentration and cooling gives crystals of sulphur.




