Acids, bases and salts Worksheet-4
Multiple Choice Questions:
(A) It is fully ionized in water producing H+ ions.
(B) It is partially ionized in water producing H+ ions.
(C) Concentrated acetic acid is strong.
(D) It does not ionize in water.
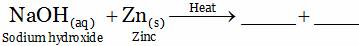
(A) NaZnO2 + H2(g) (B) ZnO + H2O
(C) NaH + ZnO (D) None
(A) 8-9 (B) 6-7 (C) 4-6 (D) 2-5
(A) Acidic (B) Basic (C) Neutral (D) Strong acidic
(A) Anode
(B) Cathode
(C) Both anode and cathode in small amounts
(D) Both A and C
(A) NH3, NaCl (B) NaCl, NH3, CO2, H2O
(C) NaOH, HCl, CO2, H2O (D) NH4Cl, NaOH, CO2
(A) on heating it decomposes evolving Hydrogen gas
(B) on heating it decomposes forming sodium ions (Na+)
(C) On heating it decomposes evolving carbon di-oxide gas.
(D) A, B, C
(A) On treating with dilute acids (di. H2SO4 / HCl) chlorine is produced
(B) On reaction with water oxygen is produced which has bleaching property.
(C) Chlorine present in calcium oxychloride has bleaching property.
(D) Both a and b
Answer key:
Acetic acid is a weak acid because if undergoes partial ionization in water thus producing a small amount of hydrogen ions.
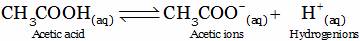
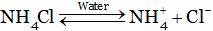 Solution is acidic due to the formation of
Solution is acidic due to the formation of