Acids, bases and salt Worksheet-5
Multiple Choice Questions:
(A) Salt of strong acid and weak base
(B) Salt of strong base and weak acid
(C) Salt of strong acid and strong base
(D) Salt of weak acid and weak base
(A) HNO3 (B) HCl(conc) (C) H2SO4
(D) HCl(conc) (E) HF
(A) 2 (B) 7 (C) 6 (D) 7.4
(A) 1M HCl
(B) 1M CH3COOH
(C) Both of them will have same Ph
(D) None of these
(A) NaOH, H2CO2 (B) NaH, H2CO3
(C) H2CO3, NaOH (D) All of these
Reason-Washing soda reacts with moisture forming sodium hydroxide.
(A) Both assertion and reason are correct
(B) Assertion is correct but reason is wrong
(C) Assertion is wrong but reason is correct
(D) Both assertion and reason are wrong.
(A) CH3COO– + H+ (B) H+ + OH–
(C) Both a and b (D) CH3CO– + H+
(A) They are basic in nature and react with copper evolving Hydrogen gas.
(B) They contain acids which can react with copper metal of vessel forming poisonous metal compound.
(C) Water present in the food reacts with copper forming copper hydroxide.
(D) The given statement is wrong they can be kept in copper vessels
(A) Metal salt + water (B) Metal salt + H2 gas
(C) Metal salt + CO2(g) (D) Metal salt + O2 gas
(A) Lime water turns milky due to the formation of calcium carbonate(s)
(B) White ppt of calcium carbonate dissolves due to the formation of calcium hydrogen carbonate.
(C) Carbon di-oxide reacts with calcium hydroxide forming calcium oxide which makes lime water milky.
(D) None of these
(A) (A)
(B) (B)
(C) Fizzing will be same in both the test tubes.
(D) All of these
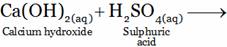
(A) Ca(SO4)2 + H2O (B) CaSO4 + H2O + H+(aq)
(C) CaSO4 + H2O + OH–(aq) (D) CaSO4 + 2H2O
(A) Alkaline as it increases the pH of mouth and protects teeth.
(B) Acidic as it lowers the pH of the mouth thus destroying microorganisms in mouth which causes tooth decay.
(C) Neutral as change in pH of the mouth will cause damage to the tissues.
(D) None of these
(A) acidic (B) basic (C) Neutral (D) Strong base
(A) Anode
(B) Cathode
(C) Both anode and cathode in small amounts
(D) Both A and C
(A) Sodium carbonate, carbon dioxide and water
(B) Sodium oxide, carbon di-oxide and water.
(C) sodium hydroxide and water
(D) None of these.
(A) sodium hydrogen carbonate
(B) sodium hydrogen carbonate + tartaric acid
(C) sodium hydrogen carbonate + acetic acid
(D) Sodium hydrogen, carbonate + citric acid.
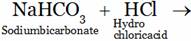
(A) NaCl + H2C3 (B) N2CO3 + 2HCl
(C) NaCl + H2O + CO2 (D) NaCl + H2O + CO
Answer key:
Copper sulphate is the salt of copper hydroxide and sulphuric acid.
Hydrofluoric acid cannot be stored in glass bottles. Glass is made of sodium silicate (Na2SiO3). silicate bond can only be cleaved by Hydro fluoric acid
Hydrochloric acid is a strong acid where as acetic acid is a weak acid.
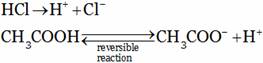 H+ concentration insolution is less
H+ concentration insolution is less
When exposed to air washing soda (Na3CO3) absorbs water forming Na2CO3.10 H2O.10 H2O
Hydrochloric acid is a mineral acid and citric acid is an organic acid. Mineral acids are stronger than organic acids. The amount of H+ ions produced is more in test tube (A)
Na2CO3 → 2Na+ + CO32–The solution is strongly basic due to the formation of sodium hydroxide. H2O → H+ + OH–
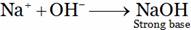
Baking powder contains sodium hydrogen carbonate and tartaric acid. If only NaHCO3 is taken, on heating NaHCO3 will form Na2CO3 which will give bitter taste to Bakery products. So tartaric acid is mixed. Na2CO3 formed reacts with tartaric acid and is neutralized and gives a pleasant taste
Metal carbonates react with acids forming their salts, water and carbon di-oxide gas.