Atoms and Molecules Worksheet-4
A. An atom of carbon (C-12)
B. 1/12th of mass of carbon atom (C-12)
C. 1/12th Of hydrogen atom.
D. One atom of all the elements.
A. 32 times B. 12 times
C. 8/3 times D. 12/32 times
A. 6 × 1022 B. 6 × 1023 C. 12 × 1023 D. 1.2 × 1023
A. 100 g B. 50 g C. 200 g D. 5 g
A. 1g of CO2 B. 1g of N2
C. 1g of H2 D. 1g of CH4
A. Dalton B. Berzelius C. Proust D. Lavoisier.
A. Any element B. Any chemical compound
C. Pure chemical compound D. None of these.
A. 4.0 g B. 6.0 g C. 64.0 g D. 16.0 g
A. 11.0 g B. 22.0 g C. 4.4 g D. 44 g
A. Rutherford B. Dalton C. Bohr D. Einstein
Answer:
Explanation: Relative atomic mass is expressed in units known as a.m.u. It is simply represented as u (unified mass). An a.m.u is defined as the mass of one twelfth (1/12) of the mass of one atom of carbon taken as 12.
Explanation: Atomic mass of sulphur = 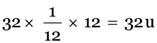
32/12 = 8/3 times.
An atom of sulphur is 8/3 times heavier than an atom of carbon.
Explanation: 44 g of CO2 has oxygen atoms = 2 × 6.022 × 1023
4.4 g of CO2 has oxygen atoms 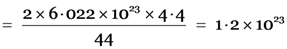
Explanation: 6.022 × 1023 Ca2+ and CO32– ions are present in CaCO3 = 100 g
3.01 × 1023 Ca2+ and CO32– ions are present in CaCO3 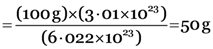
Explanation: 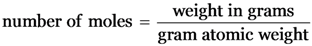
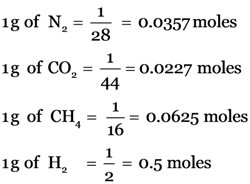
1 mole of a substance has 6.022 × 1023 particles
1 g of H2 (0.5 mol) has max. no. of molecules i.e. 3.011 × 1023 molecules.
Explanation: Fact
Explanation: Law of constant proportion: A pure chemical compound always consists of the same elements that are combined together in a fixed (or definite) proportion by mass.
Explanation: In compound CS2
12 g of carbon combine with 64 g sulphur
3 g of carbon will combine with 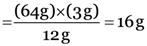 sulphur
sulphur
Explanation: 6.022 × 1023 molecules of CO2 are present in 44g of CO2
3.011 × 1023 molecules of CO2 will be present in = 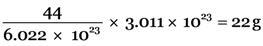
Explanation: Fact