Carbon and its compounds Worksheet-7
Fill in the blanks:
The property of self combination is called _____.
If diamond is burnt in oxygen _____ gas is evolved.
Diamonds are used as drilling equipments because they are _____.
______ is the name of first member of homologous series of compounds of general formula CnH2n+2O.
The next higher homologue of chloropentane is _____.
The difference in molecular masses of C4H9OH and C9H15OH is ______.
Alkanes under go ______ reactions. (substitution/addition)
Multiple-Choice Question:
(A) Toluene (B) Water (C) Benzene (D) H2SO4
(E) Both a & c
(A) Heptanoic acid (B) Heptan-3-oic acid
(C) Octanoic acid (D) Octanal
(A) Hexanone (B) Hexanal (C) Hexene (D) Hexyne
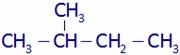
(A) Isopentane (B) 2-methylbutane
(C) 3-methylbutane (D) 2-methylpentane
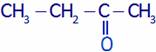
(A) Ethyl methyl ketone (B) Butanal
(C) Butanone (D) Butanol
(A) 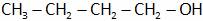
(B) 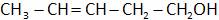
(C) 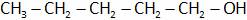
(D) 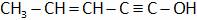

(A) 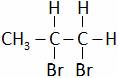 (B)
(B) 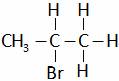
(C) 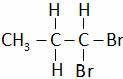 (D) All of these
(D) All of these
(A) Chloro butane + HCI (B) di-chloro butane
(C) Tri-chloro butane (D) None of these
Answer key
Catenation
Carbon dioxide
Very hard/extremely hard/hard
Methanol
Chlorohexane
42/ forty two/Forty-two
Substitution
(E)
(C)
(B)
(B)
(C)
(C)
(A)
(B)