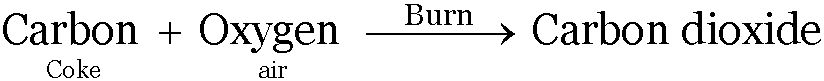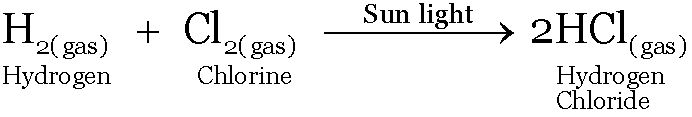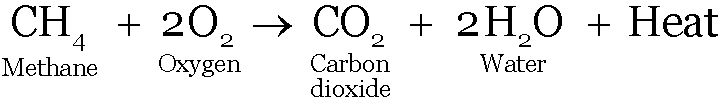COMPOUNDS
-
A compound is a substance made up of two or more elements chemically combined in a fixed proportion by mass.
For example water (H2O) is a compound made up of two elements, hydrogen and oxygen.
Hydrogen and oxygen are present in the ratio of 2: 16 or 1: 8 by mass. In pure form of water form any source the two elements are present in same fixed ratio i.e. 1 : 8 by mass.
Types of Compounds:
-
The compounds are classified into two types based on the source of origin. These are:
-
Inorganic compounds
-
Organic compounds
Inorganic compounds:
-
These compounds are obtained from non-living sources such as rocks and minerals.
Example:
-
Common salt, Marble, Washing soda, Baking soda, Carbon dioxide, Ammonia, Nitric acid, Hydrochloric acid
Organic compounds:
-
The organic compounds are the compounds obtained from living beings i.e., plants and animals
-
All the organic compounds contain carbon as their essential constituent. The organic compounds are quite often known as carbon compounds.
Example:
-
cooking gas, ethane, acetone, alcohol, acetic acid, Sugar, protein, oil
-
Compounds are also classified based upon their characteristics as:
1. Acids
2. Bases
3. Salts
Sulphuric acid, Hydrochloric acid, Nitric acid, Carbonic acid etc
Sodium hydroxide, Potassium hydroxide, Calcium hydroxide, Magnesium hydroxide etc
Salts are generally formed by chemical chemical combination of acids and bases dissolved in water.
-
Sodium hydroxide + Hydrochloric acid
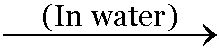 Sodium chloride + Water
Sodium chloride + Water
-
Sodium chloride, Calcium nitrate, Zinc sulphate etc
Characteristics of Compounds:
A pure compound is composed of the same elements irrespective of the source
-
In a compound the same elements are always present in fixed ration by mass irrespective of the source.
For example-
-
In carbon dioxide, two elements carbon and oxygen are present in the ratio of 3: 8 by mass.
A pure compound is homogenous in nature
A chemical compound is formed by the chemical reaction between the constituent elements
For example:
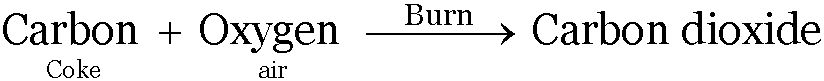
Properties of compounds are altogether different from the constituent elements
-
A compound is formed as a result of chemical reaction between the elements. Water formed by the reaction between Hydrogen and Oxygen has different properties.
-
Water is a liquid whereas hydrogen and oxygen is a gas.
-
Hydrogen gas is combustible and Oxygen gas is a supporter of combustion but water is neither combustible nor supports combustion.
-
Water stops combustion and is used as fire extinguisher.
Constituents of chemical compounds cannot be separated by physical methods of separation
-
Hydrogen and oxygen gas can be separated from water by passing electric current (electrolysis). It cannot be separated by mechanical methods like filtration, distillation etc.
Example:
-
When the mixture of iron filings and sulphur powder is heated, a black compound known as iron sulphide (FeS) is formed.
-
Compound cannot be separated into its components by physical methods.
Iron filings can be separated from the mixture of iron filings and sulphur by a magnet but iron cannot be separated from iron sulphide by a magnet.
Energy is either released or absorbed during the preparation of a compound.
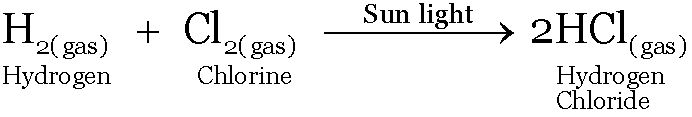
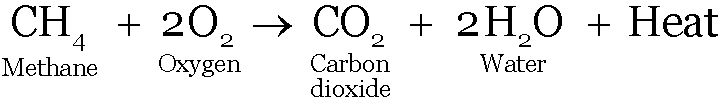
 Sodium chloride + Water
Sodium chloride + Water