IONS
-
An ion is a positively or negatively charged atom (or group of atoms).
Cations:
-
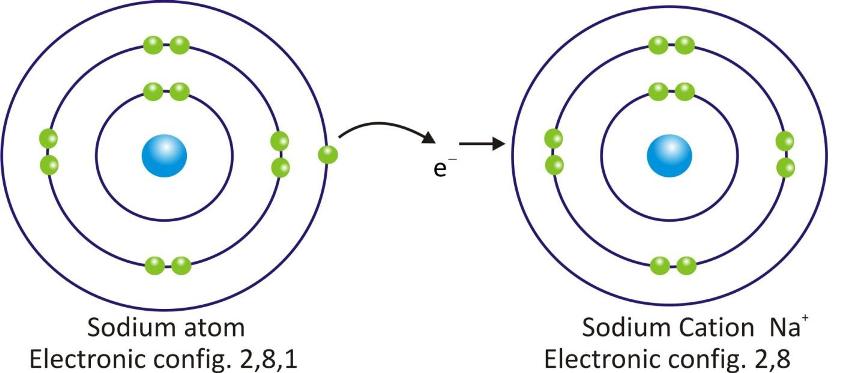
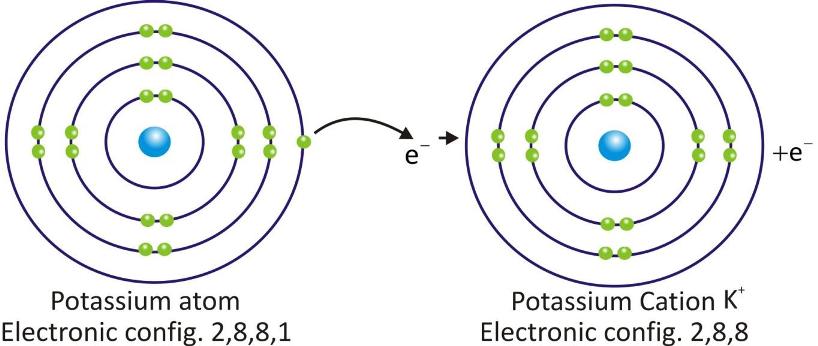
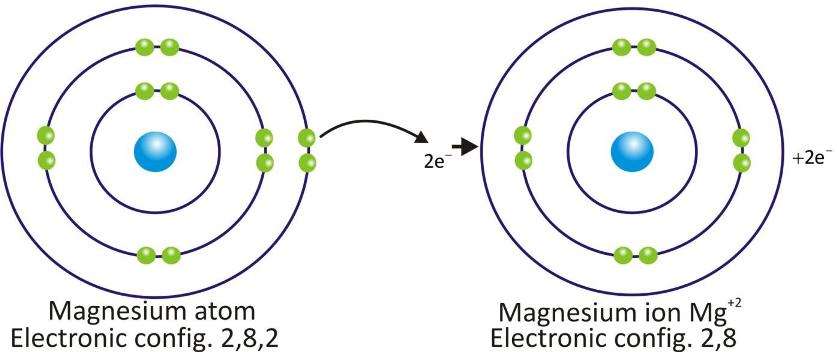
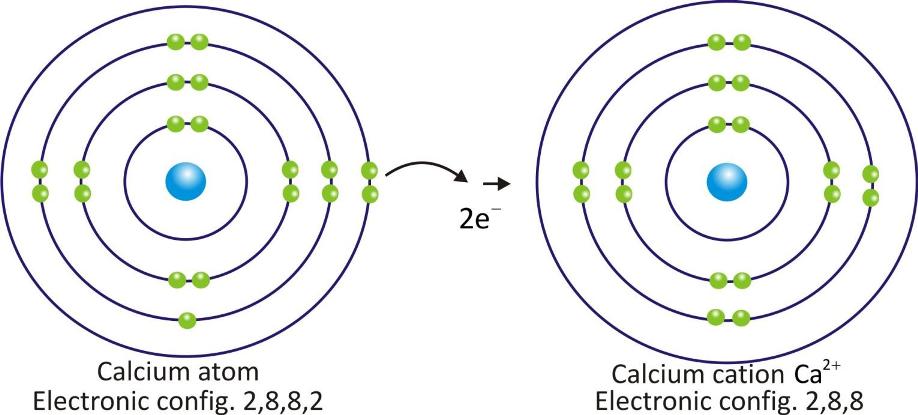
Cations are positively charged species and are formed when a metal atom loses electrons.
-
Cations are represented by symbol of element and positive sign as superscript (A+).
Sodium Cation:
Potassium Cation:
Magnesium Cation:
Calcium cation:
Anions:
-
Anions are negatively charged species and are formed when the atom of an element gains electrons.
-
Anions are represented by symbol of element and negative sign as superscript (A–).
Example:
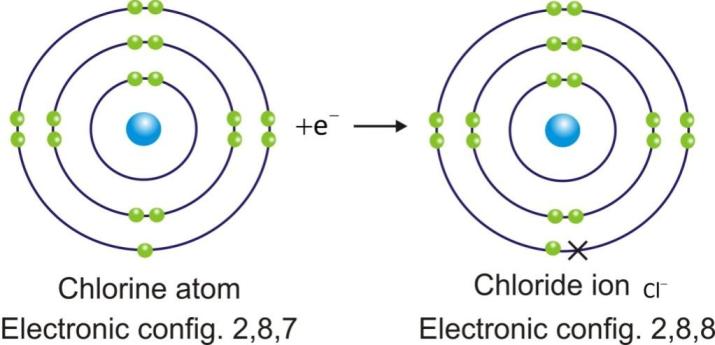
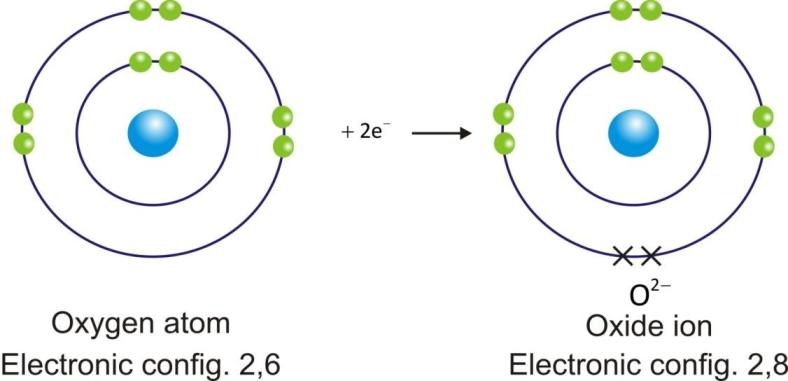
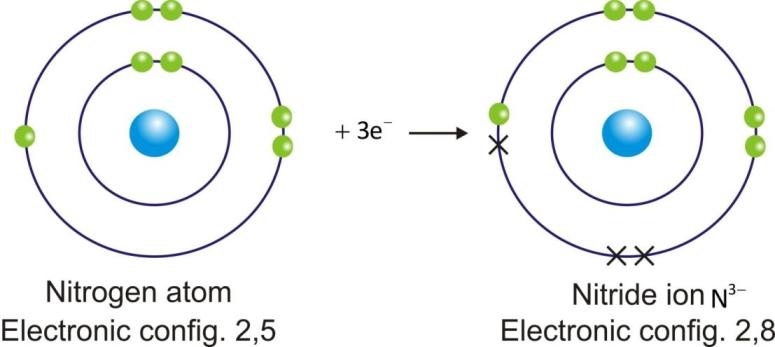
Ionic Compounds:
-
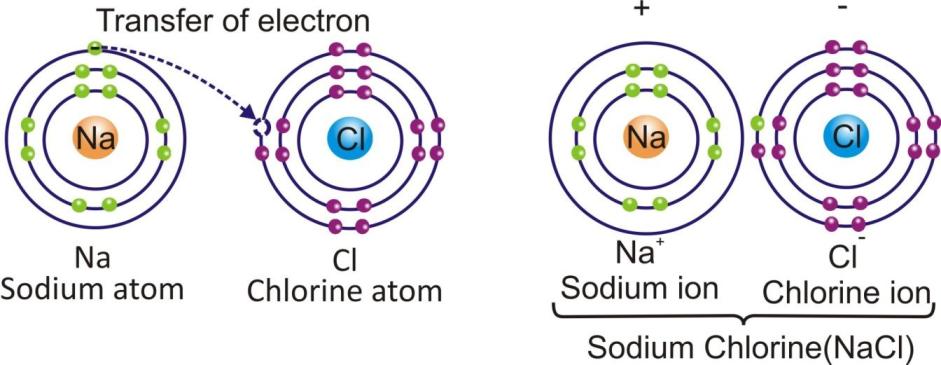
The compounds which are made up of ions are known as ionic compounds.
-
In an ionic compound, the positively charged ions (cations) and negatively charged ions (anions) are held together by the strong electrostatic forces of attraction.
Sodium chloride Na+ + Cl– → NaCl
Formation of calcium chloride CaCl2
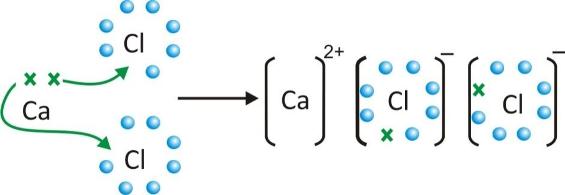
-
The force which hold the ions together in an ionic compound is known as ionic bond or electrovalent bond.
For example:
-
sodium chloride (NaCl) is an ionic compound.








