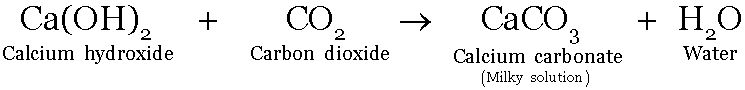SEPARATION OF CONSTITUENTS OF A MIXTURE
-
Mixtures are formed by mixing of pure substances i.e. elements and compounds
-
The constituents of a mixture do not react. Mixture shows the properties of its constituents.
-
Many naturally occurring substances are associated with some impurities. These impurities are removed by suitable method or technique.
The method of separation of constituents from a mixture depends upon-
1. The type of mixture (homogeneous or heterogeneous)
2. The nature of the substances present in the mixture.
Separation of constituents from a heterogeneous mixture:
-
Heterogeneous mixtures are comparatively easy to separate because the different constituents can be easily identified and have different physical properties.
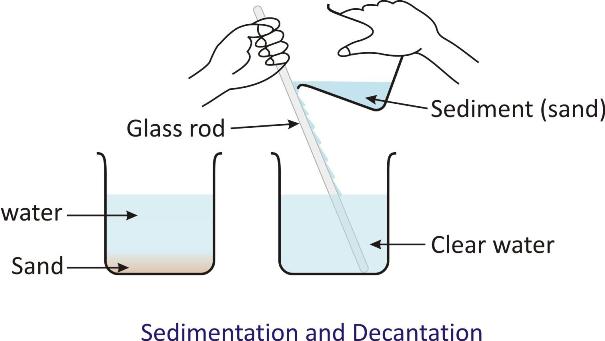
Sedimentation followed by decantation and filtration:
-
This method is useful when the constituents are to be separated from a suspension.
For example:
Separation of sand from the mixture of sand and water
Separation of calcium carbonate from its suspension
-
Suspension of calcium carbonate is formed by bubbling carbon dioxide gas through lime water (calcium hydroxide) taken in flask or beaker
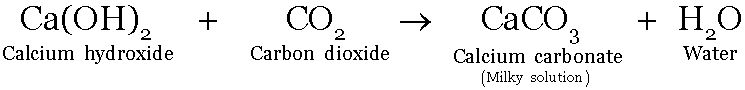
-
The suspension is milky in colour. White residue of calcium carbonate can be separated from the suspension by the process of sedimentation followed by filtration.
-
The suspension is allowed to remain undisturbed for sometime in a beaker or any other container. The particles settle down under the influence of gravity. The settling of the particles is called sedimentation.
-
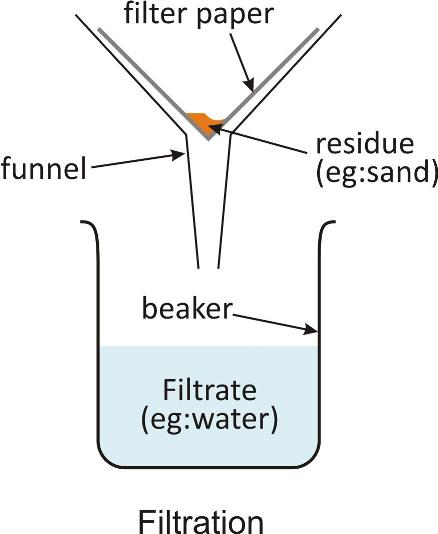
The white residue of calcium carbonate formed can be separated by filtration.
-
The solid and liquid component of solution can be separated by this method.
Mixtures which can be separated by sedimentation and filtation
-
Separation of crystals of copper sulphate, nitre (potassium nitrate) or potash alum prepared by some suitable methods.
-
The white precipitate of silver chloride formed by mixing solutions of silver nitrate and sodium chloride can be separated by filtration.
-
A mixture of iron particles and sulphur can also be separated by this process. The mixture is dissolved in carbon disulphide solvent. Sulphur dissolves in carbon disulphide and passes into the solution while iron particles remain as such. The solution is filtered, solid iron remains on the filter paper and solution is collected in the beaker. Sulphur can be collected from the solution by evaporation.
Separation of constituents of mixtures using centrifugal machines:
Centrifugal machines:
-
Help in the formation of precipitates in mixtures.

Centrifuge
For example
-
Centrifugal machines are commonly used in diagnostic centres where tests are performed.
-
Washing machines used for washing dirty clothes are centrifugal machines in nature
Centrifugation of blood
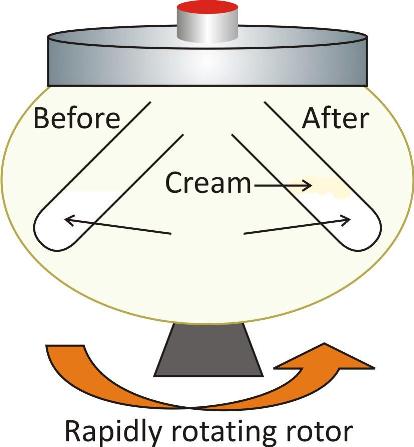
Separation of Cream from Milk
-
A large quantity of soluble fat is present in the milk. With the help of centrifugal machine fat can be separated from milk as cream.
Process:
-
The given sample of milk is placed in the machine which is rotated at a very high speed by passing electric current.
-
The particles of fats present collide with one another at a fast speed.
-
As a result, they combine to form bigger particles in the form of precipitate or residue known as cream. It keeps on escaping from the outlet that is provided leaving behind the fat free milk.
-
Single or double toned milk is prepared by this method.
Magnetic separation:
-
The mixture which contains magnetic components can be separated by magnets.
For example
-
Iron filings and sulphur present in a mixture can also be separated with the help of a horse shoe magnet.
-
This mixture is placed in a dish and the magnet is repeatedly moved over the mixture.
-
The iron filings stick to the magnet in each operation and can be removed.
-
After sometime, the entire iron filings present in the mixture will be removed leaving behind sulphur which is of non-magnetic nature.

Separation of iron filings by magnet
Separation of substance undergoing sublimation from a mixture:
-
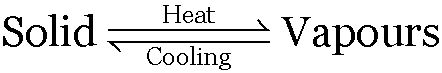
The process of sublimation is used to separate those solids from their mixtures which directly pass into the vapour state upon heating without passing through the liquid state and the vapours on cooling change to the solid state again
For example:
-
Separation of substances like naphthalene, camphor, ammonium chloride, benzoic acid, iodine etc. from the non-volatile components present in the mixture.
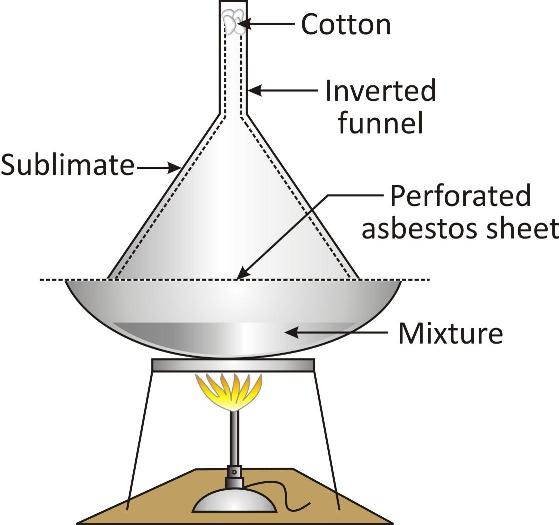
Process:
-
The mixture is taken in a china dish and is covered by a perforated porcelain plate.
-
An inverted glass funnel is placed over the dish and its stem is plugged with cotton.
-
The dish is heated gently when the volatile substance changes into the vapours. These vapours pass through the perforations of the plate and get collected on the inner cold surface of the funnel. This is known as sublimate.
-
The nonvolatile constituents remain on the dish. The sublimate can be removed from the funnel.
Examples:
-
A mixture of ammonium chloride (undergoes sublimation) and sodium chloride (does not undergo sublimation) can be separated.
-
A mixture of camphor which is a solid with pleasant smell (undergoes sublimation) and salt can be separated.
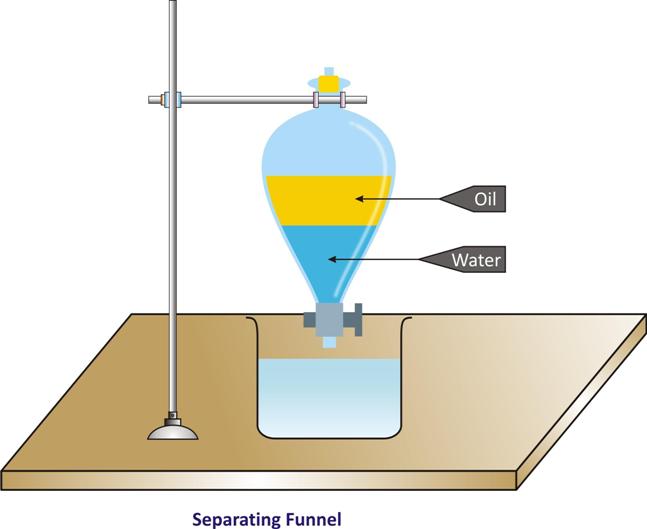
Separation of two immiscible liquids:
-
Oil is insoluble in water. Oil and water form separate layers when placed together. These are therefore, known as immiscible liquids.
-
The mixture of oil and water is heterogeneous and can be separated by separating funnel.
Example:
-
A liquid mixture of mustard oil and water can be separated. Mustard oil being lighter will form the upper layer while water being heavier will form the lower layer.
-
A liquid mixture of carbon tetrachloride (forms lower layer) and water (forms, upper layer) can also be separated.