Structure of the atom Worksheet-5
(i) What is the name given to the number of protons in the nucleus of the atom?
(ii) What is the name given to the number of protons plus number of neutrons in the nucleus of the atom?
(i) What is the atomic number of the element?
(ii) What is the mass number of the element?
(iii) Name the element and give its electronic configuration.
(iv) Predict the valeney of the element.
Calculate the percentage of each present in it.
Answer:
Mass number of the element = No. of protons (15) + No. of neutrons (16)
= 15 + 16 = 31
Representation of the element = 15X31
The element with atomic number (Z = 15) is phosphorus (P). It is represented as 15P31
∴ Atomic no. of oxygen (Z) = 8
(ii) No. of protons in the nucleus plus no. of neutrons in the nucleus of an atom is known as mass number.
∴ Mass no. of oxygen (A) = 8 + 8 = 16
(ii) The mass no. of element = No. of protons + No. of neutrons
= 9 + 10
= 1
(iii) The element with Z = 9 is fluorine (F).
Its electronic configuration : 2, 7.
(iv) The valiancy of fluorine is 1 and is calculated as 8 – 7 = 1.
No. of electrons = No. of protons =16
No. of neutrons = Mass no. — no. of protons
= 32 – 16 = 16
Electronic arrangement = K(2), L(8), M(6)
Valency of the element = 8 – 6 = 2
Atomic number of atom M = 10 + 2 = 12
No. of protons in atom M = 12
Mass number of atom M = No. of protons + No. of neutrons =12 +12 = 24
The element M with atomic number 12 is magnesium (Mg).
∴ the percentage of B – 11 isotope = 100 – x
The average atomic mass of boron
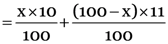
But the given average atomic mass of boron = 10.8 u
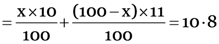
10x + 1100 – 11x = 10.8 × 100 = 1080
X = 1100 – 1080 = 20
∴ Percentage Abundance of B-10 isotope = 20%
Abundance of B – 11 isotope = 80%
∴ The percentage of 29Cu65 isotope = 100 – x
From the above data, the relative atomic mass of Cu
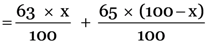
But the given relative atomic mass of Cu = 63.5 u
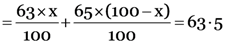
63x + 6500 – 65x = 6350
–2x = 6350 – 6500 = –150
2x = 150
or x = 75u
Percentage of 29Cu63 isotope = 75%
Percentage of 29Cu65 isotope = 25%.