Chemical reaction and equations worksheet-7
One Word questions:
A piece of wood is crafted into a shape which allows a batsman to apply force on the ball. What type of change occurs in wood?
An iron rod can be magnetized by passing an electric current through an insulated coil wound around. What type of change occurs in Iron rod?
When a person eats chocolate what type of change occurs?
What type of change is tarnishing of silver cutlery?
What type of change takes place on ignition of gasoline?
Mass of the individual substances that undergo the change, always, either increases or decreases. What type of change is it?
It is temporary change and in most cases it can be reversed by the reversal of conditions what type of change is it?
What type of change is melting of an ice-cream ?
What type of change occurs when Sugar dissolved in tea?
What type of change occurs in wood on burning it in air?
What type of change occurs on cleaving water molecule?
What type of change occurs on crushing an aspirin?
What type of change occurs when milk becomes sour if kept for a long time ?
What type of change occurs on Burning of Lighter fluid ?
What type of change occurs when egg is boiled?
KCl is the chemical formula Potassium Chloride.
What is the name of Ag2O ?
What is the product formed when electric current is passed through Hydrogen and Oxygen gas in a container?
What is the product formed when Copper metal is dipped in Silver nitrate solution?
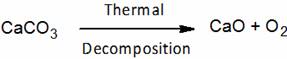 is an example of –
is an example of –
Answer key:
Physical or Physical change
Physical or Physical change
Chemical or Chemical change
Chemical or Chemical change
Chemical or Chemical change
Chemical or Chemical change
Physical or Physical change
Physical or Physical change
Physical or Physical change
Chemical or Chemical change
Chemical or Chemical change
Physical or Physical change
Chemical or Chemical change
Chemical or Chemical change
Chemical or Chemical change
Potassium Chloride
Silver oxide
Water or H2O
Copper nitrate
Decomposition reaction