Atoms and Molecules Worksheet-8
-
What is the mass of 0.2 mole of oxygen atoms?
-
Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur.
-
Calculate the number of aluminium ions in 0.051 g of aluminium oxide (Al2O3).
-
The mass of an atom of element (X) is 2.0 × 10–23 g. Calculate its atomic mass.
-
Calculate the molar mass of Nitric acid.
-
What is number of molecules present 1.5 mole of ammonia (NH3) ?
-
Explain why the number of atoms in one mole of hydrogen gas is double the number of atoms in one mole of helium gas.
-
If the valency of carbon is 4 and that of sulphur is 2, what is the chemical formula and name of the compound formed between carbon and sulphur atoms?
-
A mole is quite often known as chemist’s. dozene. Why it is so named ?
-
The mass of one molecule of a substance is 4.65 × 10–23 g. What is its molecular mass? The compound is made up of carbon and oxygen. Name the compound
-
An element forms an oxide A2O5.
(i) What is the valency of the element A?
(ii) What will be the formula of the chloride of the element?
-
On analyzing an impure sample of sodium chloride, the percentage of chlorine was found to be 45.5. What is the percentage of pure sodium chloride in the sample?
-
What weight of calcium contains the same number of atoms as are present in 3.2 g of sulphur ?
Answer:
-
a) 0.2 mole of oxygen atoms
Mass of 1 mole of oxygen (O) atoms = 16 u
Mass of 0.2 mole of oxygen (O) atoms = 0.2 × 16 = 3.2 u
-
Gram molecular mass of sulphur (S8) = 8 × gram atomic mass of sulphur
= 8 × 32 g = 256 g
No. of S8 molecules in 256 g of sulphur = 6.022 × 1023
No. of S8 molecules in 16 g of sulphur 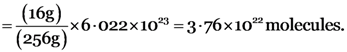
-
Step I : Number of moles of Al2O3 in 0.051 g of Al2O3
Gram molecular mass of Al2O3 = 2 × gram atomic mass of Al + 3 × gram atomic mass of I = (2 × 27 g) + (3 × 16 g) = 102 g
Now, 102 g of Al2O3 = 1 mol
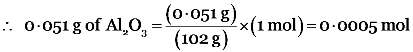
Step II : Calculation of no. of Al ions in 0.0005 mole of Al2O3
1 mole of Al2O3 contain Al atoms = 2 × N0
0.0005 mole of Al2O3 contain Al atoms = 2 × 0.0005 × N0
= 2 × 0.0005 × 6.022 × 1023 = 6.022 × 1020 atoms
In Al2O3 the valency of Al = 3 +
No. of aluminium ions (Al3+) present is the same as the no. of Al atoms
∴ No. of Al3+ ions = 6.022 × 1020 ions.
-
One atom of element (X) has mass = 2.0 × 10–23 g.
6.022 × 1023 atoms of element (X) will have mass
= (2.0 × 10–23 g) × (6.022 × 1023)
= 12.044 ≈ 12.0 g
-
Chemical formula of Nitric acid is (HNO3)
Molar mass of HNO3 = (1 × atomic mass of H) + (1 × atomic mass of N) + (3 × atomic mass of O)
= (1 × 1u) + (1 × 14u) + (3 × 16u) = 63u
-
1.0 mole of ammonia (NH3) contains = 6.022 × 1023 molecules
1.5 mole of ammonia (NH3) contain 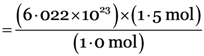
= 9.033 × 1023 molecules.
-
Hydrogen gas is diatomic in nature (H2) while helium gas is monoatomic (He). As a result, the number of atoms in one mole of hydrogen is (2 × NA) which is double as compared to number of atoms in one mole of helium (NA)
-
The chemical formula of compound can be written by exchanging the valencies (cross-over). Therefore, the expected formula is C2S4 or CS2. The compound is called carbon disulphide.
-
A dozon represents a fixed number of articles i.e. 12. Similarly, a mole represents a fixed number of particles i.e. Avogadro’s number (NA) or 6.022 × 1023
-
Given: Mass of one molecule of substance = 4.65 × 10–23 g
Mass of 6.022 × 1023 molecules of substance = 6.022 × 1023 × (4.65 × 10–23) = 28 g
The compound is Carbon monoxide (CO)
Molecular weight (12u + 16 u = 28 u) Gram molecular weight = 28g
-
Formula of oxide of the element = A2O5
(I) The valency of the element A in the oxide = 5
(ii) The formula of its chloride will be ACl5
-
Molecular mass of pure NaCl = Atomic mass of Na + Atomic mass of Cl
= 23 + 35.5 = 58.5u
Percentage of chlorine in pure 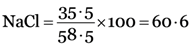
if chlorine is 60.6 parts, NaCl = 100 parts
if chlorine is 45.5 parts, 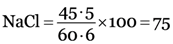
Thus, percentage of pure sodium chloride in the sample is 75%.
-
Step I. Number of atoms in 3.2 g of sulphur
Gram atomic mass of S = 32 g
32 g of sulphur contain = 6.022 × 1023 atoms
3.2 g of sulphur contain 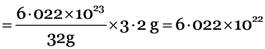 atoms
atoms
Step II. Weight of 6.022 × 1022 atoms of calcium
Gram atomic mass of Ca = 40 g
6.022 × 1023 atoms of Ca weigh = 40 g
6.022 × 1022 atoms of Ca weigh 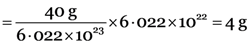 .
.
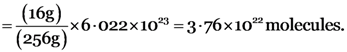
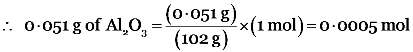
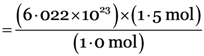
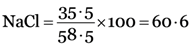
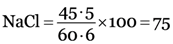
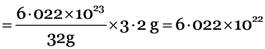 atoms
atoms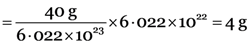 .
.