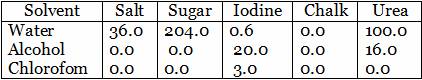Is matter around us pure Worksheet-9
-
State one property in which a solution of sugar in water resembles a mixture of sugar and sand and one property in which it differs from it.
-
Account for - Fresh air cannot be regarded as a pure substance.
-
The table given below shows number of grams of five different solids dissolving in 100 g of the solvents : water, alcohol and chloroform (all at 20°C).
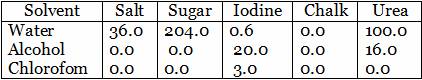
(a) Which solid dissolves best in water at 20°C ?
(b) Which solid is maximum soluble in alcohol ?
(c) Which solid is insoluble in all three solvents ?
-
Butter is an example of one type of colloidal solution. Name it. Give a reason for your choice.
-
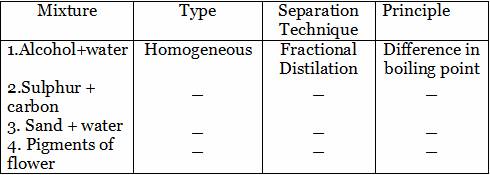
Complete the following based on separation techniques. The first one is done for you.
-
Some solids dissolve easily while in liquids the others do not
(a) What is the name given to the liquids which dissolve solids ?
(b) What is the name given to the clear liquid formed when a solid dissolves in a liquid ?
(c) What is the name given to the liquid which contains in it some suspended panicles ?
-
Butter is an example of one type of colloidal solution. Name it. Give a reason for your choice.
Answer:
-
Resemblance: Both are mixtures and taste sweet.
Difference: Solution of sugar and water is homogeneous whereas sugar and sand is heterogeneous mixture.
-
Fresh air is a mixture of gases like nitrogen, oxygen, carbon dioxide, water vapours etc. Since it is not a single substance, it is not pure.
-
(a) Sugar is best soluble in water at 20°C
(b) Iodine is maximum soluble in alcohol.
(c) Chalk is insoluble in all the three solvents.
-
Butter is an example of colloidal solution in which liquid is dispersed in solid. It is also called gel. Reason for the choice. On pressing butter, liquid drops come out of it leaving behind a solid. This clearly shows that butter is a gel.
-
1. Homogeneous, Fractional Distilation, Difference in boiling point
2. Homogeneous, Evaporation, Difference in physical states
3. Hetereogeneous, Filtration, Difference in solubility in water
4. Homogeneous, Chromatography, Difference in adsorption of different components.
-
(a) The liquids are known as solvents
(b) The clear liquid is called solution or true solution.
(c) The liquid is known as suspension.
-
The colloidal solution is an example in which solid acts as the dispersion medium while liquid as the dispersed phase. It is also called gel.
Reason for the choice. On pressing butter, liquid drops come out of it leaving behind a solid. This clearly shows that butter is a gel.