CONCENTRATION OF A SOLUTION
Mass percent:
If A and B are the two components of a binary solution-
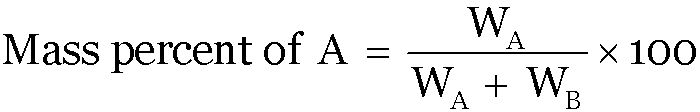
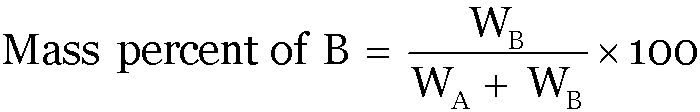
Volume Percent:
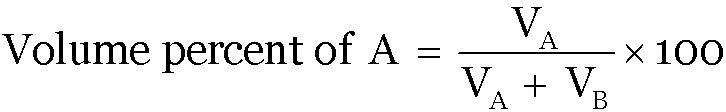
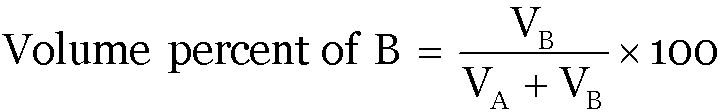
Question 1
Calculate the masses of cane sugar and water required to prepare 250 g of 25% solution of cane sugar.
Ans:
Mass percent = 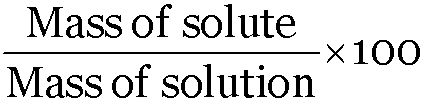
Mass percent = 25, Mass of solution = 250 g
∴ 25 = 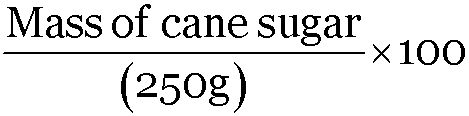
∴ Mass of cane sugar = 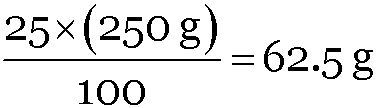
Mass of water = 250 – 62.5 = 187.5 g
Question 2
A solution has been prepared by dissolving 5 g of urea in 95 g of water. What is the mass percent of solution in water?
Ans:
Mass percent (Mass %) = 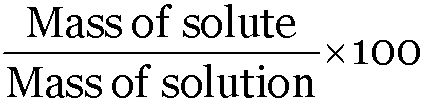
Mass of urea = 5 g, Mass of water = 95 g
Mass percent of urea = 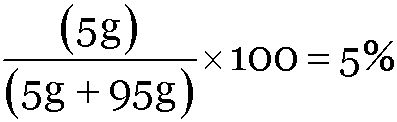
Question 3
A solution contains 5 mL of alcohol mixed with 75 mL of water. Calculate the concentration of this solution in terms of volume percent.
Ans:
Concentration of solution = 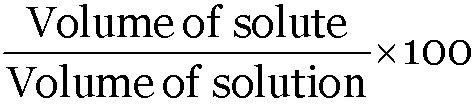
Volume of alcohol = 5 mL
Volume of solution = (5 + 75) = 80 mL
Concentration of solution = 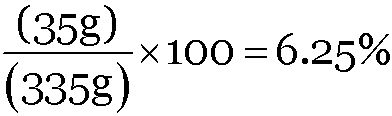
Question 4
A solution contains 35 g of common salt in 300 g of water. Calculate the concentration of the solution.
Ans:
Concentration of solution = 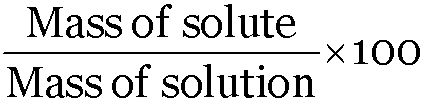
Mass of common salt = 35g
Mass of water = 300 g
Mass of solution = (300 + 35) = 335g
Concentration of solution = 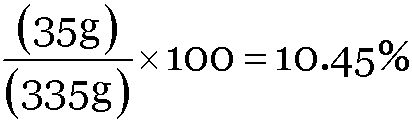 .
.