SUBLIMATION
-
The change of state directly from solid to gas without changing into liquid is known as sublimation.
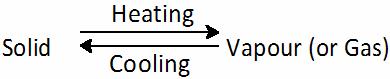
-
Solid carbon dioxide is stored under high pressure when the pressure is reduced to 1 atmosphere it changes to gaseous state.
-
The common substances which undergo sublimation are:
Ammonium chloride
Iodine
Camphor
Naphthalene
Anthracene
-
There are weak intermolecular forces of attraction between the particles of these compounds so they can easily change from their solid to gaseous state. Very less energy is required to overcome the force of attraction between the particles.
Effect of Change of Pressure:
-
The physical state of matter can also be changed by increasing the pressure or decreasing the pressure.
-
Gases can be changed into liquids by increasing the pressure.
-
And some solids like solid carbon can change into gases by decreasing the pressure.
Liquefaction of gases:
-
When pressure is increased and temperature is lowered gases can be liquefied. The molecules of gases are in a state of continuous rapid motion with negligible force of attraction between them. Every molecule has almost independent existence.
-
When pressure is increased and temperature is lowered the kinetic energy of the molecules decreases the molecules come closer because they are unable to resist the attractive forces that start operating between them.
-
The molecules come closer and closer thus changing into liquid state.
Plasma:

-
Plasma is a state of matter gas in which a certain portion of the particles are ionized.
-
Heating a gas may ionize its molecules and atoms (reduce or increase the number of electrons in them), thus turning it into plasma, which contains charged particles: positive ions and negative electrons or ions. Ionization can be induced by other means, such as strong electromagnetic field applied with a laser or microwave generator.
-
The fluorescent tube and neon sign bulbs consist of plasma.
-
Inside a neon sign bulb there is neon gas and inside a fluorescent tube there is helium gas or some other gas. The gas gets ionized, that is, gets charged when electrical energy flows through it.
-
This charging up creates a plasma glowing inside the tube or bulb. The plasma glows with a special colour depending on the nature of gas.
-
The Sun and the stars glow because of the presence of plasma in them.
-
The plasma is created in stars because of very high temperature.

