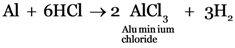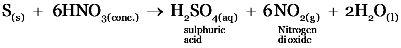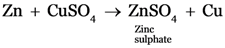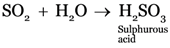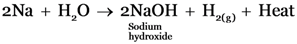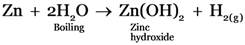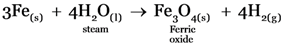Materials: Metals and non-metals Worksheet-8
-
Give reason: Immersion rods for heating liquids are made up of metallic substances.
-
Give reason: Copper cannot displace zinc from its salt solution.
-
What happens when
(a) Dilute sulphuric acid is poured on copper plate?
(b) Iron nails are placed in copper sulphate solution?
Write word equations of the reactions involved.
-
Choose appropriate words from the brackets and complete the statements.
Noble gases are found in (free state/compound forms).
-
Choose appropriate words from the brackets and complete the statements.
Potassium after combustion will form (acidic oxide/basic oxide).
-
Choose appropriate words from the brackets and complete the statements.
(Iodine/bromine) has antiseptic properties.
-
Choose appropriate words from the brackets and complete the statements.
German silver has (copper/silver) as major constituent.
-
Explain with equation what happens when
(a) Hydrochloric acid is poured on aluminium foils.
(b) Hot concentrated nitric acid is poured on powdered sulphur.
(c) Sodium is placed on water.
(d) Zinc granules are kept in copper sulphate solution.
(e) Sulphur dioxide is dissolved in water.
-
From among the set of metals — sodium, zinc, iron, copper, silver, select the following giving equations for :
Two metals which will liberate hydrogen from water.
-
From among the set of metals — sodium, zinc, iron, copper, silver, select the following giving equations for :
One metal which will displace copper from copper sulphate solution.
-
From among the set of metals — sodium, zinc, iron, copper, silver, select the following giving equations for :
One metal which will not displace copper from copper sulphate solution.
Answer:
-
Metals are good conductors of heat and electricity hence immersion rods used for heating liquids are made up of metallic substances.
-
Copper is less reactive than zinc hence it cannot replace zinc from its salt solution.
-
(a) Copper will dissolve as air will be present. Dilute acids like HCl/ H2SO4 has no effect on copper in the absence of air.
Assuming air is present copper will dissolve in H2SO4.
2Cu + 2H2SO4 + O2 → 2CuSO4 + 2H2O
(b) Iron will replace copper from copper sulphate the colour of solution will become green due to the formation of Ferrous sulphate.
Fe + CuSO4 → FeSO4 + Cu
-
Noble gases are found in free state. Noble gases are inert hence are found in free state.
-
Potassium after combustion will form basic oxide. Metals form basic oxide.
-
Iodine has antiseptic properties.
-
German silver has copper as major constituent.
Composition of German silver:
Cu 25%, Zn 30%, Ni 40%
-
(a)
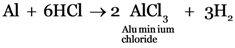
(b) 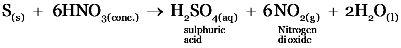
(c) 2Na + 2H2O → 2NaOH + H2(g)
(d) 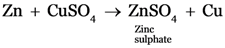
(e) 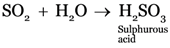
-
Sodium, Zinc and iron will liberate Hydrogen from water as they are present above hydrogen in the metal reactivity series.
Sodium reacts with cold water vigorously, Iron reacts with steam and zinc reacts with boiling water.
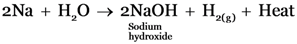
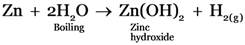
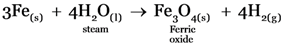
-
Sodium, Zinc, iron all will displace copper from copper sulphate solution.
2Na + CuSO4 → Na2SO4 + Cu
Zn + CuSO4 → Na2SO4 + Cu
Fe + CuSO4 → FeSO4 + Cu
-
Silver will not displace copper from copper sulphate solution.