Atoms and Molecules Worksheet-7
(i) Al2(SO4)3 (ii) CaCl2 (iii) K2SO4
(iv) KNO3 (v) CaCO3.
(a) Quick lime (b) Hydrogen bromide
(c) Baking powder (d) Potassium sulphate.
Answer:
∴ 0.024 g of Mg = 6.022 × 1020 atoms
(ii) Calcium chloride
(iii) Potassium sulphate
(iv) Potassium nitrate
(v) Calcium carbonate.
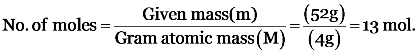
No. of particles (atoms) = No. of moles (n) × Avogadro’s no. (No)
= (0.1) × (6.022 × 1023) = 6.022 × 1022 atoms.
6.022 × 1023 atoms of carbon have mass = 12 g
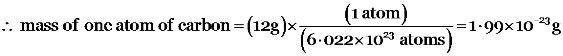
For example-
(i) Carbonate (CO3)2–ion
(ii) Nitrate ion (NO3–)
(iii) Ammonium ion (NH4)+
(iv) Phosphate ion (PO4)3–
(a) Quick lime : It is the commercial name of the compound. Its chemical name is calcium oxide and the chemical formula is CaO.
Elements present : calcium (Ca) : oxygen (O)
(b) Hydrogen bromide : The chemical formula of the compound is HBr
Elements present : hydrogen (H); bromine (Br)
(c) Baking powder : It is the commercial name of the compound. Its chemical name is sodium hydrogen carbonate and the chemical formula is NaHCO3
Elements present: sodium (Na), hydrogen (H), carbon (C), oxygen (O).
(d) Potassium sulphate : The chemical formula of the compound is K2SO4
Elements present: potassium (K), sulphur (S), oxygen (O).
Elements present : calcium (Ca) : oxygen (O)
Elements present: sodium (Na), hydrogen (H), carbon (C), oxygen (O).
The chemical formula of the compound is K2SO4
Elements present: potassium (K), sulphur (S), oxygen (O).