Atoms and Molecules Worksheet-1
A. 32 g B. 16 g C. 48 g D. 8 g
A. H2S B. HS C. HS2 D. H3S
A. NH4SO3 B. (NH4)2SO3
C. NH4SO4 D. (NH4)2SO4
A. Na(CO3)2 B. NaHCO3 C. Na2CO3 D. NaCO3
A. CaPO4 B. Ca3(PO4)2 C. Ca2(PO4)3 D. Ca(PO4)2
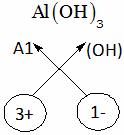
A. AlOH B. Al(OH)2 C. Al(OH)3 D. Al(OH)4
A. 10 u B. 18 u C. 20 u D. 16 u
A. 32 u B. 64 u C. 48 u D. 28 u
A. 96 u B. 78 u C. 90 u D. 64 u
A. 90 u B. 98 u C. 92 u D. 82 u
Answer:
Explanation: The formula of water is H2O
2g of hydrogen combines with 16 g oxygen
4g of hydrogen will combine with 
Explanation:
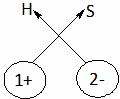
Hydrogen sulphide
H2S
Explanation: Ammonium sulphate
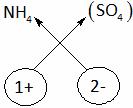
Explanation: Sodium carbonate
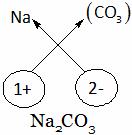
Explanation: Calcium phosphate
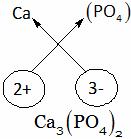
Explanation: Aluminium hydroxide
Explanation: Water (H2O)
Molar mass of H2O = (2 × atomic mass of H) + (1 × atomic mass of O) = (2 × 1u) + (1 × 16u) = 18u
Explanation: Sulphur dioxide (SO2)
Molar mass of SO2 = (1 × atomic mass of S) + (2 × atomic mass of O) = (1 × 32u) + (2 × 16u) = 64u
Explanation: mmonium carbonate (NH4)2CO3
Molar mass of (NH4)2CO3 = (2 × atomic mass of N) + (2 × (4 × atomic mass of H) + (atomic mass of C) + (3 × atomic mass of O)
Molar mass of (NH4)2CO3 = (2 × 14u) + (2 × (4 × 1u) + (12u) + (3 × 16u) = 96u
Explanation: Sulphuric acid (H2SO4)
Molar mass of (H2SO4) = (2 × atomic mass of H) + (1 × atomic mass of S) + (4 × atomic mass of O) + (2 × 1u) + (4 × 16u) = 98u