Chemical reaction and equations worksheet-5
Multiple Choice Questions:
(A)Solid, gas liquid, aqueous (B) Gas, solid, liquid, aqueous
(C) Aqueous, liquid, gas, solid (D) Solid, liquid, gas, aqueous
(A) Combination reaction (B) Photodecomposition
(C) Redox reaction (D) Displacement reaction
(A) CaCO3 (B) Ca(OH)2 (C) CaO (D) CoCl2
(E) CaSO4
(A) Yellow (B) Blue (C) Black (D) Red–brown
(A) FeSO3 (B) FeSO2 (C) FeSO4 (D) FeS
(A) 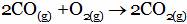
(B) 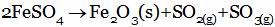
(C) 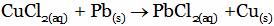
(D) 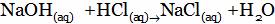
(A) Combination reaction (B) Endothermic reaction
(C) Precipitation reaction (D) Color of reaction mixture changes
(A) Lead Oxide (B) Nitrogen di-Oxide
(C) Oxygen(D) Lead nitrate (D) Lead nitrate
(A) Pb+3, NO3– (B) Pb+, NO2+ (C) Pb+4, NO+ (D) Pb+2, NO
(E) Pb2+, NO3–
(A) Hydrogen gas and ferrous chloride are produced
(B) Chlorine gas and ferric hydroxide are produced.
(C) Ferric chloride and water are produced
(D) No reaction takes place.
Answer keys:
(D)
(B) Silver chloride dissociates into silver and chloride using light energy.

(C) Calcium oxide on reaction with water gives calcium hydroxide or slaked lime. Slaked lime is applied on the wall of buildings.
(D) A red- brown coating of copper metal is formed on the surface of ironnails.
(C) FeSO3 is ferrous sulphite, FeSO2 the formula is wrong, and FeS is ferrous sulphide. Valency of iron is 3 or 2, Sulphate is 2
(C)
(A) As sulphur-di-oxide combines with oxygen forming sulphur –tri-oxide.
(B) 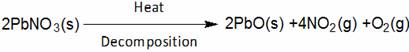
(E) Pb+4 plumbic ion, NO+ Nitronium ion
(A) Fe + 2HCl → FeCl2 + H2(g)