Acids, Bases and Salts Worksheet-3
(A) Sodium hydrogen carbonate
(B) Water
(C) Vinegar
(D) None of these
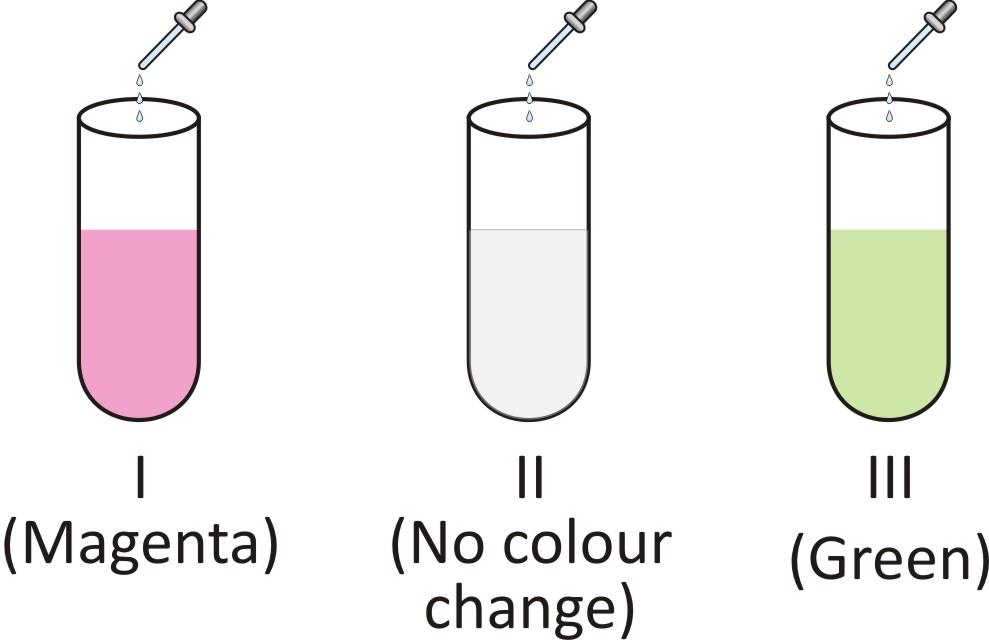
(A) I: Sugar solution; II: Lime water; III: Lime juice
(B) I: Sugar solution; II: Lime juice; III: Lime water
(C) I: Lime water; II: Sugar solution; III: Lime juice
(D) I: Lime juice; II: Sugar solution; III: Lime water
Column I Column II
(Common name) (Chemical formula)
(a) Slaked Lime (i) KOH
(b) Caustic soda (ii) Mg(OH)2
(c) Caustic potash (iii) Ca(OH)2
(d) Milk of magnesia (iv) NaOH
(A) (a)–(iii), (b)–(iv), (c)–(i), (d)–(ii)
(B) (a)–(iii), (b)–(iv), (c)–(ii), (d)–(i)
(C) (a)–(i), (b)–(ii), (c)–(iii), (d)–(iv)
(D) (a)–(iv), (b)–(i), (c)–(iv), (d)–(iii)
(A) Acidic (B) Basic (C) Neutral (D) None of these
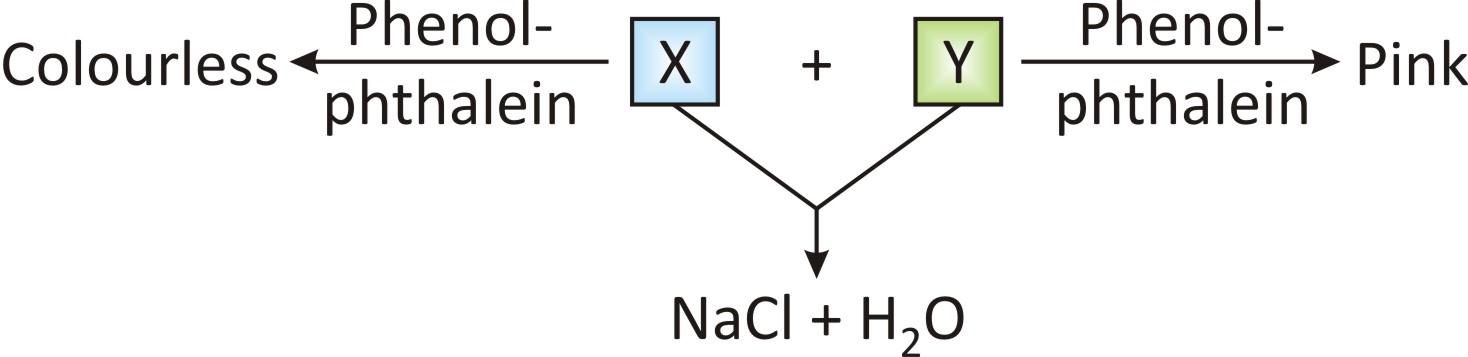
(A) X: NaOH; Y: NaCl (B) X: HCl; Y: NaOH
(C) X: NaOH; Y: HCl (D) X: HCl; Y: NaCl
Statement 1: Acids are sour in taste while bases are bitter in taste.
Statement 2: Baking soda does not taste sour.
(A) Both statements 1 and 2 are true and statement 2 is the correct explanation of statement 1
(B) Both statements 1 and 2 are true but statement 2 is not the correct explanation of statement 1
(C) Statement 1 is true, and statement 2 is false
(D) Both statements 1 and 2 are false.
(A) Colour
(B) Physical state
(C) Temperature
(D) Pressure
(A) Glass containers are transparent
(B) Glass containers are cheaper
(C) Metal containers are not easily available
(D) Metal reacts with the acid stored in them
(A) NaCl + H2O → NaOH + HCl
(B) CaCO3 + H2O → Ca(OH)2 + CO2
(C) NaOH → HCl → NaCl + H2O
(D) CuSO4 + Zn → ZnSO4 + Cu
Set–I: (i) HCl, (ii) H2SO4, (ii) HNO3
Set–II: (i) CH3COOH, (ii) HCOOH, (iii) C2H5COOH
(A) Set–I consists of mineral acids while set–II consists of organic acids
(B) Set–I consists of organic acids while set–II consists of mineral acids
(C) Both sets consists of mineral acids.
(D) Both sets consist of organic acids
Answer Key: