Sources of energy Worksheet-12
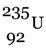 .
.(ii) Starting from 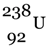 , describe the chain of nuclear reactions that leads
, describe the chain of nuclear reactions that leads 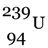 as the final product. What is the significance of this reaction?
as the final product. What is the significance of this reaction?
b. Why it is difficult to use hydrogen as a source of energy?
c. What is the maximum temperature attained in a concave reflector type solar cooker?
Answer:
One of these is a heavy group of nuclei with mass numbers ranging from 130 to 149, e.g., Barium and Lanthanum.
The other is a lighter group of nuclei with mass numbers ranging from 85 to 104, e.g., Krypton and Molybdenum.
(i) In prompt fission, the heavy nucleus disintegrates the moment it is bombarded.
(ii) In delayed fission, a projectile (e.jg., neutron) enters the target nucleus and causes an instability which results in the fission of the target nucleus after a short while.
(iii) Spontaneous fission is a form of radioactive decay where an atom's nucleus splits into two smaller nuclei and generally one or more neutrons. Spontaneous fission generally in atoms with atomic numbers above 90.
(iv) Spontaneous fission is a relatively slow process except for the heaviest isotopes. For example, uranium–238 decays by alpha decay with a half-life on the order of 109 years, but also decays by spontaneous fission on the order of 1016 years.
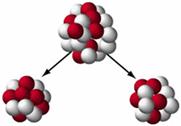
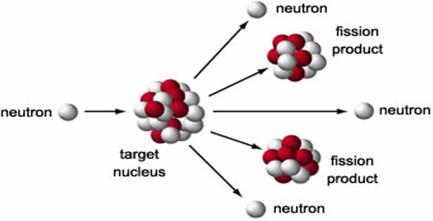
The four process that can place after neutrons are emitted in a fission reaction are :
(i) At least one such neutron hits another nucleus to cause its fission releasing more neutrons.
(ii) The neutrons may be captured by other nuclei without causing their fission.
(iii) The neutrons may be captured by other nuclei (present either in the fissionable material or in the surrounding container without causing fission).
(iv) The neutrons may not interact with other nuclei and may escape the system.
The energy of released neutrons is lowered from 2 MeV to 0.025 MeV by passing them through a moderator.
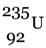 . FP stands for fission product.90
. FP stands for fission product.90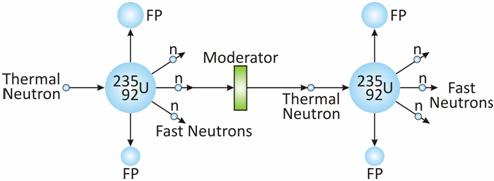
(ii) (a) 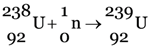
(b) 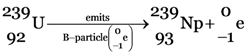
(c) 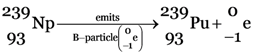
The significance of this chain reaction is that 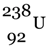 , which is not easily fissionable is converted into
, which is not easily fissionable is converted into 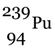 (plutonium-239) which can be fissioned easily.
(plutonium-239) which can be fissioned easily.
b. It cannot be stored safely as (i) it bums with explosion and (ii) has low ignition temperature.
c. About 200°C.