Metals and non metals Worksheet-4
Multiple-Choice Question (One Option Correct):
(A) Acidic (B) Basic (C) Amphoteric (D) A & B
(E) B and C
(A) Na2O (B) Na2O2 (C) NaO (D) NaO2
(A) Exothermic (C) Endothermic
(C) No heat change occurs. (D) Depends on the state of metal
(A) Mg(OH)2 (B) MgOH
(C) Mg2+ + H+ + OH+– (D) MgO(aq) + H2O(l)
(A) Acidic (B) Basic (C) Amphoteric (D) B and C
(A) CuO (B) Cu2O (C) CuO2 (D) All of these
(A) FeO (B) Fe2O3 (C) Fe3O4 (D) Fe2O3.XH2O
(A) In water it forms Aluminium hydride (AlH3) which looses H+ ions.
(B) It reacts with base giving salt and water.
(C) Both (A) and (B)
(D) It turns blue litmus red .

(A) ZnCl2(aq) + H2O(l) (B) ZnCl2(aq) + H+ + OH–
(C) Zn2Cl + H2O(l) (D) ZnCl + H2O(l)
(A) Ca(OH)2 formed is lighter than water so it floats on the surface.
(B) Bubbles of hydrogen gas formed stick to the surface of metal.
(C) CaO formed floats on the surface.
(D) All of these
Answer key:
(A)
(A)
(A)
(A) 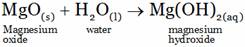
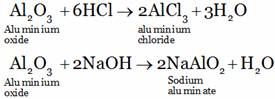
(C) Aluminium oxide reacts with acid as well as base forming salt and water
(A) Copper forms copper (II) oxide on prolonged heating in air.
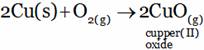
(C) Iron reacts with the oxygen of air on heating to form iron (I,II) Oxide.
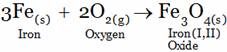
(B) 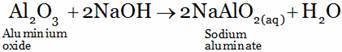
(A) 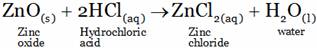
(B) Ca(s) + 2H2O → Ca(OH)2(aq) + H2(g)