MOLECULES
-
A molecule is the smallest particle of a substance (element or compound) which has the properties of that substance and can exist in the Free State.
-
It is an electrically neutral group of two (or more) atoms chemically bonded together.
-
The force which holds the atoms together in a molecule is called bond.
Molecules of Elements:
-
The molecule of an element consists of two (or more) similar atoms chemically combined together.
For example:
-
A molecule of Nitrogen element contains 2 Nitrogen atoms combined together, and it is written as N2.
-
Nitrogen gas consists of N2 molecules and not of atoms of Nitrogen.
-
Hydrogen gas exists as H2 molecules, oxygen gas as O2 molecules and chlorine gas as Cl2 molecules.
-
The number of atoms present in one molecule of an element is called its atomicity.
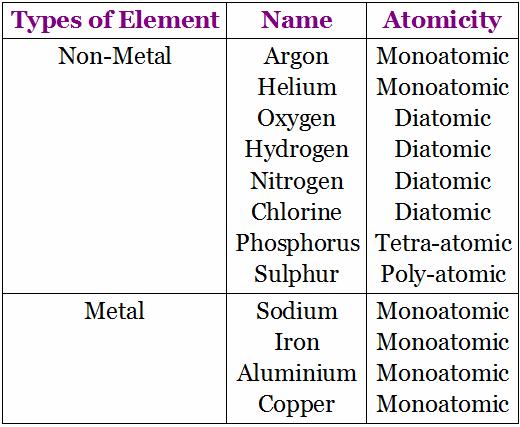
Atomicity of some elements (redraw)
-
The atomicity of some of the common elements is:
-
Noble gases (He, Ne, Ar and Kr) the atomicity is 1.
-
The atomicity of hydrogen, nitrogen, oxygen, chlorine, bromine and iodine is 2 each.
-
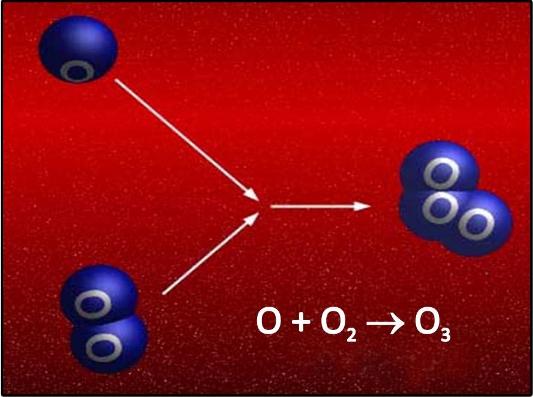
The atomicity of ozone is 3.
-
The atomicity of phosphorus is 4.
-
The atomicity of sulphur is 8.
Molecules of Compounds:
-
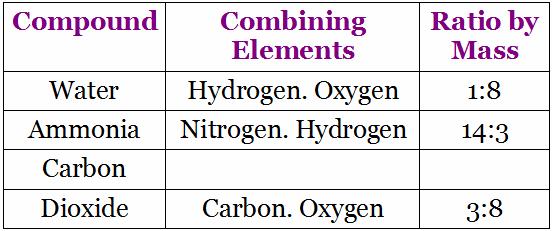
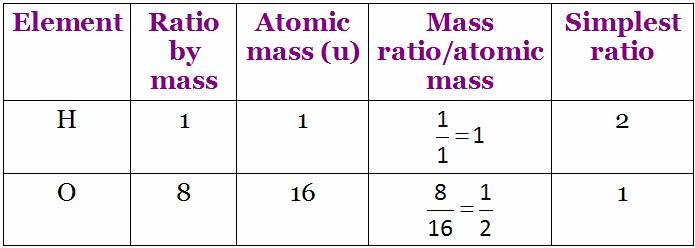
The molecule of a compound contains two (or more) different types of atoms chemically combined together in a simple whole number ratio and it is the smallest entity which has all the properties of a compound.
For example:
-
Hydrogen chloride or Hydrochloric acid contains atoms of Hydrogen and chlorine.
-
Water is a compound. A molecule of water (H2O) is made up of Hydrogen and Oxygen atoms.
Molecules of some compounds (redraw)