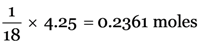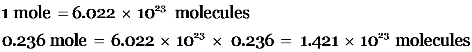Atoms and Molecules Worksheet-5
A. 6.0 × 1024 B. 6.022 × 1022
C. 6.022 × 1023 D. 6.022 × 10–23
A. 6.022 × 1010 B. 6.022 × 1022
C. 6.022 × 1023 D. 6.022 × 1021
A. Law of conservation of mass
B. Law of constant proportion
C. Dalton’s Atomic theory.
D. Avogadro’s Law.
A. 0.5 mol B. 2.0 mol C. 1.5 mol D. 0.75 mol
A. Atoms are indivisible.
B. Atoms combine in simple whole number ratios.
C. All atoms of an element may not have same mass.
D. Atoms of different elements have different masses.
A. 40 B. 30 C. 48 D. 36
A. FeSO4 B. Fe(SO4)2 C. Fe3(SO4)2 D. Fe2(SO4)3
A. Dalton’s atomic theory
B. Law of constant composition
C. Law of multiple proportions.
D. Law of conservation of mass.
A. 100 B. 10 C. 0.01 D. 0.1
A. 1.5 × 1023 B. 2 × 1023 C. 4 × 1023 D. 6 × 1023
Answer:
Explanation: Fact
Explanation:
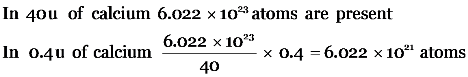
Explanation: Law of constant proportion: A pure chemical compound always consists of the same elements that are combined together in a fixed (or definite) proportion by mass.
Explanation: 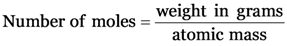
No. of moles in 60 g of calcium 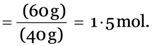
Explanation: All atoms of an element have same mass according to Dalton atomic theory.
Explanation: Percentage of Ca 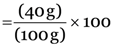 = 40 %
= 40 %
Explanation: The valency of iron in Ferric sulphate is 3. Formula Fe2(SO4)3
The valency of iron in Ferrous sulphate is 2. Formula FeSO4.
Explanation: A chemical equation is balanced according to law of conservation of mass.
Explanation: Number of moles in 0.64 g of SO2 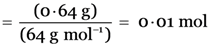 .
.
Explanation: Gram molecular weight of ammonia = 18 g
18 g ammonia = 1 mole
4.25 g ammonia =