Chemical reaction and equations worksheet-9
True or False:
BaCl2(aq) + Al2(SO4)3(aq) → AlCl3(aq) + BaSO4↓ is a displacement reaction.
The formula of Calcium nitrate is- Ca(NO3)2.
The formula of Nickel sulphide is- NiS2.
The formula of Aluminium chloride is AlCl4.
Aluminium chloride reacts with magnesium forming magnesium chloride is a displacement reaction.
2NH3(g) + H2SO4(aq) → (NH4)2 SO4(aq). Given reaction a decomposition reaction
Reduction is addition of hydrogen.
Cl2(g) + 2KBr(aq) → 2KCl(aq) + Br2(g). Given reaction is a displacement reaction.
Barium chloride reacts with Zinc sulphate forming Zinc chloride and Barium sulphate .It is an example of displacement reaction.
AgNO3(aq) + NaCl(aq) → NaNO3(aq) + AgCl(aq). Given reaction is a combination reaction.
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l). Given reaction is a combination reaction.
The name of Li3N is - lithium Nitrate.
Souring of milk is a Physical change.
Dissolving quick lime in water is an endothermic reaction.
Respiration is Endothermic process.
Baking is a chemical change.
Formula of phosphorous penta chloride is PCl3.
Oxidation is loss of oxygen.
The arrow pointing downwards ↓ along with a product represents a precipitate.
Hydrogen peroxide decomposes in the presence of light forming water and oxygen. This is an electrolytic decomposition.
The chemical equation for the formation of calcium oxide from calcium carbonate is 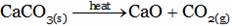
Answer key:
False: It is a double displacement reaction as ions of reactants are exchanged.
True:
False: It is NiS as the valency of Nickel is 2 and of sulphur is 2.
False: It is AlCl3 as the valency of Aluminium is 3 and Chloride is 1.
True :
False: It is a combination reaction.
True :
True :
False: It is a double displacement reaction.
BaCl2 + ZnSO4 → BaSO4 + ZnCl2
False: It is a double displacement reaction as chloride ion and Nitrate ion are exchanged.
True :
False: Its name is lithium nitride. The formula of Lithium Nitrate is LiNO3
False: It is a chemical change as the chemical composition of milk changes.
False: It is an exothermic reaction, CaO(s) + H2O(l) → Ca(OH)2 + Heat
False: Respiration is an exothermic process as energy is released.
True:
False: PCl3 is phosphorous tri chloride. Formula of phosphorous penta chloride is PCl5.
False: It is gain of oxygen.
True:
False: It is a photolytic decomposition as decomposition takes place in the presence of light.
False: CaO + O2 → CaCO3