Metals and non metals Worksheet-6
Multiple-Choice Question (One Option Correct):
Cu(s) + HCl(aq) →
(A) CuCl2 + H2(g) (B) Cu2Cl2 + H2(g)
(C) CuCl + H2(g) (D) No reaction
(A) FeCl3 + H2(g) (B) FeCl2 + H2(g)
(C) FeCl3 + H2(g) (D) No reaction
(A) Zn > Fe> Mg > Cu (B) Fe > Zn > Mg > Cu
(C) Mg > Zn > Fe> Cu (D) Cu > Mg > Zn > Fe
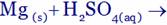
(A) MgO + H2(g) + SO2(g) (B) MgO + H2O + SO2(g)
(C) MgSO4(aq) + H2(g) (D) MgSO4(aq) + H2O(l)

(A) (Ag)2 SO4 + H2(g) (B) AgSO4 + H2O
(C) AgO + H2O + SO4(g) (D) No Reaction
(A) H2(g) (B) NO2 (C) NO (D) All of these
(A) N2O (B) NO2 (C) NO (D) All of these
(E) Both B and C
(A) 1 part - HCl(conc) and 3 part HNO3(conc)
(B) 1 part - H2SO4(conc) and 3 part HNO3(conc)
(C) 1 part - HNO3(conc) and 3 part HCl(conc)
(D) 1 part - HNO3(conc) and 3 part H2SO4(conc)
(A) Hydrogen is present in the 1st group along with Metals
(B) Hydrogen is electropositive
(C) Hydrogen can accept electrons
(D) Hydrogen is malleable.
(E) Both A and B
(A) Magnesium (B) Calcium (C) Potassium (D) Sodium
Answer key:
(D) Copper is placed below hydrogen in the metal reactivity series so it cannot displace hydrogen from acids.
(B) 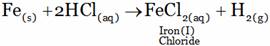
(C)
(C)
(D) Silver is a very less reactive metal it does not react with dilute acids.
(C) Nitric acid is a strong oxidizing agent, so as soon as hydrogen gas is formed it gets oxidized to water and nitric acid is reduced to N2O, NO, NO2 depending on the concentration of nitric acid.With dilute nitric acid NO gas is evolved.
(B) Nitric acid is a strong oxidizing agent, so as soon as hydrogen gas is formed it gets oxidized to water and nitric acid is reduced to N2O, NO, NO2 depending on the concentration of nitric acid. With concentrated nitric acid NO2 gas is evolved.
(C)
(B)
(C)