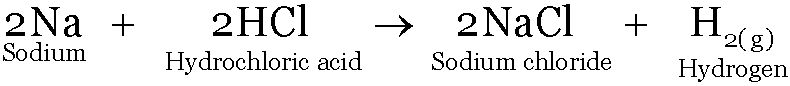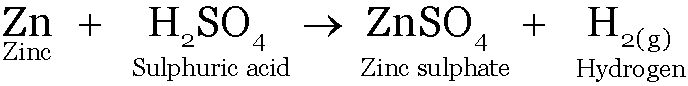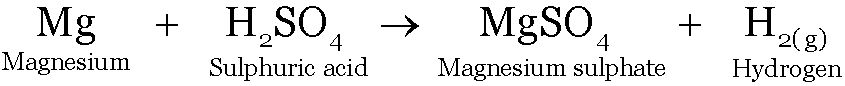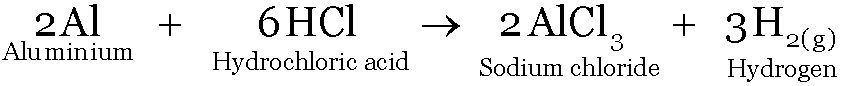REACTION OF METALS AND NONMETALS WITH ACIDS
-
Metals react with acids producing hydrogen gas. With some metals the reaction is very fast and vigorous, while with others it is slow. Hydrogen gas produced burns with a pop sound when a burning match stick is brought near to the test tube.
Example:
-
Sodium reacts with HCl to form Sodium chloride and Hydrogen.
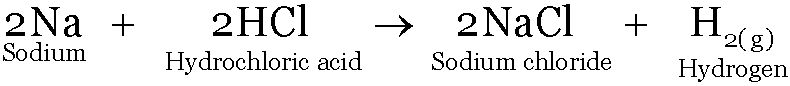
-
Zinc reacts with sulphuric acid to form Zinc sulphate and Hydrogen
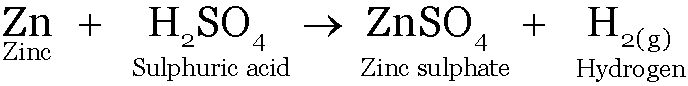
-
Magnesium reacts with sulphuric acid to form Magnesium sulphate and Hydrogen
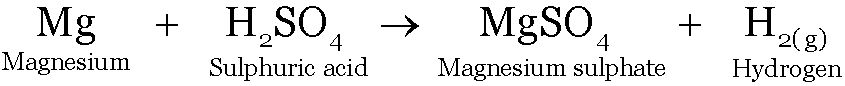
-
Aluminium reacts with Hydrochloric acid to form Aluminium chloride and Hydrogen.
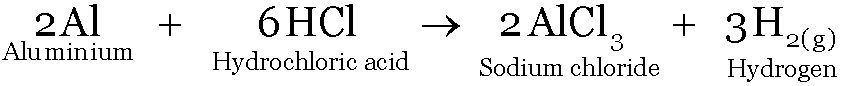
-
Non metals generally do not react with acids.