DALTON'S ATOMIC THEORY

-
John Dalton (1766-1844) developed the first useful atomic theory of matter around 1803.
-
All matter, whether an element, a compound or a mixture is composed of small particles called atoms.
-
The postulates of this theory may be stated as follows:
-
All matter is made of very tiny particles called atoms.

-
Atoms are indivisible particles, which can neither be created nor destroyed in a chemical reaction.
-
Atoms of a given element are identical in all respect of mass, shape, size and chemical properties.
-
Atoms of different elements have different masses and chemical properties.
-
Atoms combine in the ratio of small whole numbers to form compounds.
-
A chemical reaction are arrangement of atoms.
-
The relative number and kinds of atoms are constant in a given compound.
Question:
Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Ans:
The postulate of Dalton’s atomic theory that “the elements consist of atoms and that atoms can neither be created nor destroyed” is according to the law of conservation of mass.
Question:
Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Ans:
The postulates of Dalton’s atomic theory that:
(a) Atoms combine in the ratio of small whole numbers to form compounds.
(b) The relative number and kinds of atoms are constant in a given compound.
Drawbacks of Dalton’s Atomic Theory:
-
The atom is further sub divided into electrons, protons and neutrons.
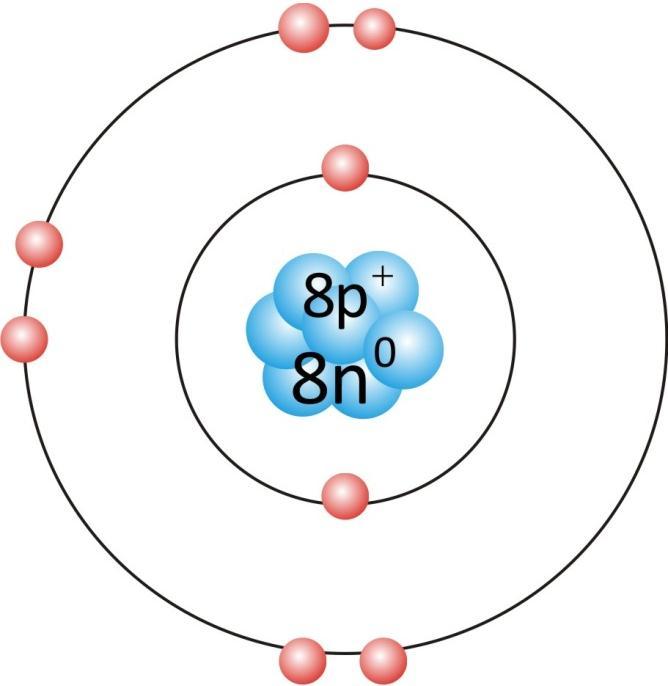
Oxygen atom
-
Atoms of same elements can have slightly different mass as in the case of Isotopes.
Isotopes of Hydrogen: Hydrogen, Deuterium, Tritium their masses are 1, 2, and 3 respectively.
-
Atoms of different elements have different masses, this fails in the case of isobars. (Isobars are the elements having same atomic mass but different atomic numbers).
18Ar40, 19K40, 20Ca40
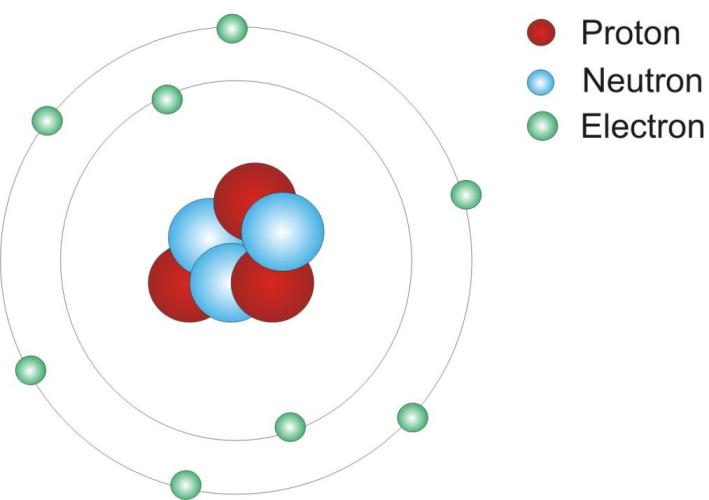
ATOM
-
An atom is the smallest particle of an element that can take part in a chemical reaction.
-
The size of an atom is indicated by its radius which is called ‘atomic radius’.
Atomic radius is measured in ‘nanometers’
1 nanometer = 1/10-9 metre
or
1 nm = 1/10-9 m
Atomic radius is measured in nanometers.
1/199 = 1 nm
1 m = 109 nm



