NONMETALS
|
Elements |
Latin names |
Symbol |
Atomic number |
Atomic weight |
Electronic configuration |
Valency = number of valence electrons or (8-number of valence electrons) |
|
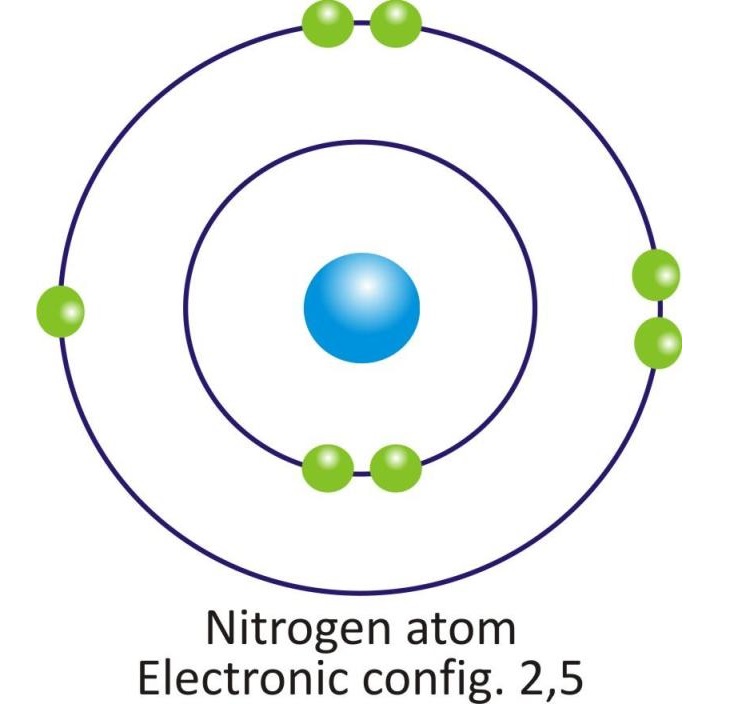
Nitrogen |
Nitrogenium |
N |
7 |
14.0067 |
2, 5 |
8 - 5 = 3 |
|
Phosphorous |
Phosphorous |
P |
15 |
30.973 |
2, 8, 5 |
8 - 5 = 3 |
|
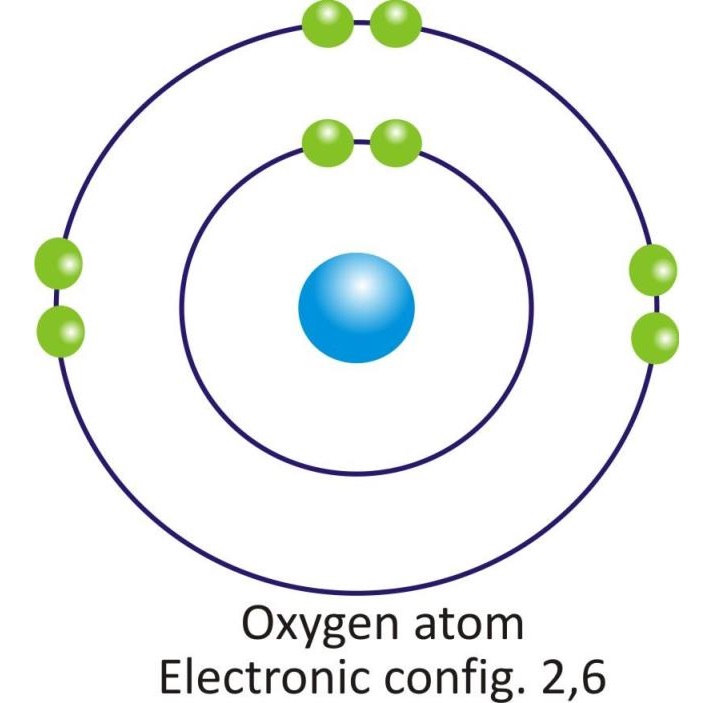
Oxygen |
Oxygenium |
O |
8 |
15.994 |
2, 6 |
8 - 6 = 2 |
|
Sulphur |
Sulphur |
S |
16 |
32.065 |
2, 8, 6 |
8 - 6 = 2 |
|
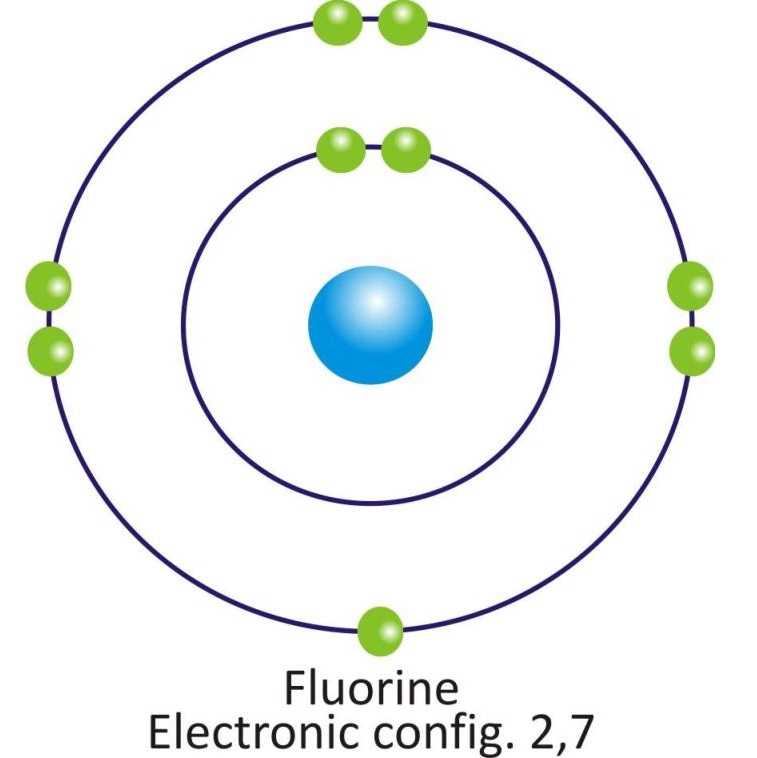
Fluorine |
Fluorum |
F |
9 |
18.99 |
2, 7 |
8 - 7 = 1 |
|
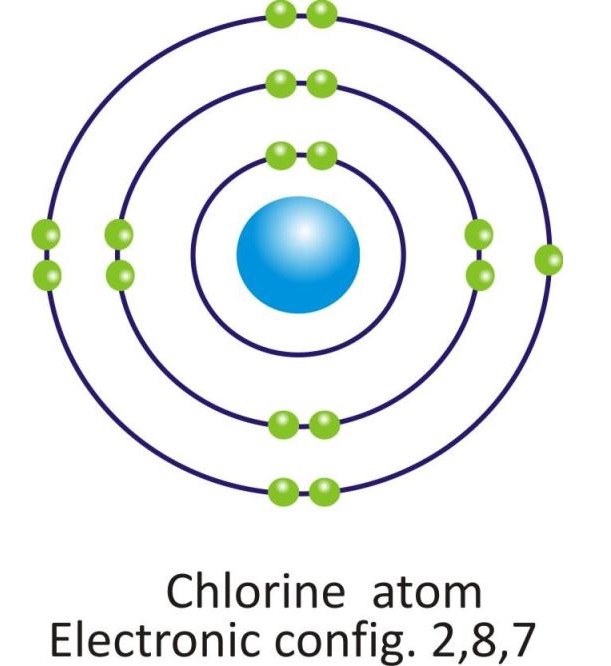
Chlorine |
Chlorum |
Cl |
17 |
35.45 |
2, 8, 7 |
8 - 7 = 1 |
|
Bromine |
Bromum |
Br |
35 |
79.904 |
2, 8, 18, 7 |
8 - 7 = 1 |
|
Iodine |
Iodum |
I |
53 |
126.904 |
2, 8, 18, 18, 7 |
8 - 7 = 1 |
Orbital diagram of atoms of some of the Nonmetals: