Basics of Chemistry Worksheet-5
(a) electron (b) proton (c) neutron (d) core
(a) electrons and nucleus (b) electrons and neutrons
(c) neutrons and protons (d) electrons and protons
(a) A chemical change needs energy. A physical change needs heat.
(b) A chemical change is easy. A physical change is hard.
(c) A chemical change is irreversible. A physical change is reversible.
(d) A chemical change is reversible. A physical change is irreversible.
(a) Rusting of an iron rod (b) Burning fuel in automobile.
(c) Making cheese out of milk (d) Evaporation of water from a cup.
(a) softness, hardness (b) sourness, sweetness
(c) acidity, alkalinity (d) alkalinity, acidity
(a) It tells if the solution is red or blue.
(b) It tells the temperature of a solution.
(c) It tells how much volume a solution has.
(d) It tells how much acidity or alkalinity a solution has.
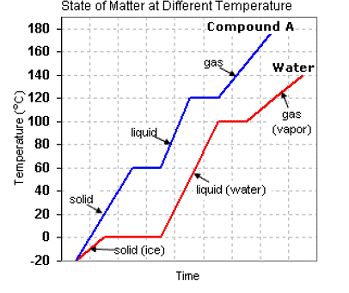
(a) 60ºC (b) 120ºC (c) −20ºC (d) 100ºC
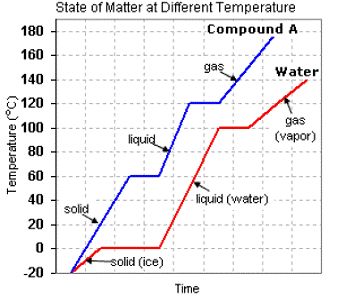
(a) Solid (Ice) (b) Liquid
(c) Gas (Vapor) (d) None of the above
(a) conduct (b) repel (c) attract (d) insulate
(a) condense (b) freeze (c) melt (d) boil
Answer Key:
(1)–(a); (2)–(d); (3)–(c); (4)–(d); (5)–(c); (6)–(d); (7)–(b); (8)–(c); (9)–(c); (10)–(a)