Carbon and its compounds Worksheet-5
Fill in the blanks:
Carbon can form long chains due to its ____ property.
In diamond, each carbon atom is bonded to _____ carbon atoms.
There are _____ Pentagonal rings in Buckminster fullerene.
_______ is the general name of the class of compounds having general CnH2n–2.
The next higher homologue of Heptyne is _______.
The name of functional group present in CH3 – CH = CH – CH3 is ____.
_____ burn in air with a yellow sooty flame.
Multiple-Choice Question:
(A) Of its ball like structure (B) It is soft and greasy
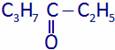 is:
is:(A) Ethyl propyl ketone (B) Pentanone
(C) Hexanone (D) Heptanone
(A) C2H5COC3H7 (B) C5H11CHO
(C) C2H5COC2H5 (D) None of these
(A) Pentene (B) Pentane (C) Pentyne (D) Cyclopentane
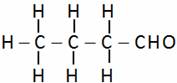
(A) Propanal (B) Butanal (C) Pentanal (D) Propyl aldehyde
(A) 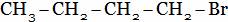
(B) 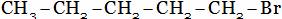
(C) 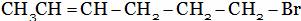
(D) 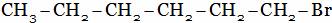
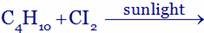
(A) 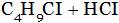 (B)
(B) 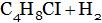
(C) 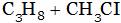 (D) All of these
(D) All of these
(A) Ethyl chloride + HCI (B) Chloro propane + HCI
(C) di-chloro propane + HCI (D) Tri-chloro propane + HCI
Answer key:
Catenation
4/ four
12/ twelve
Alkynes/Alkyne
Octyne
Alkene/Alkenes
Unsaturated hydrocarbons/Unsaturated hydrocarbon
(A)
(C)
(C)
(C)
(B)
(D)
(A)
(B)