Chemical effects of electric current Worksheet-7
-
How are bridges and automobiles prevented from rusting?
-
Why are LEDs more useful for testing the flow of electric current ?
-
Give the construction of LED.
-
After the electroplating of a spoon with silver, it was found that the anode has become thin. Why?
-
If the current in the circuit is small, how can we test its presence?
-
Explain the process of electroplating of copper.
-
During electrolysis of water, why does hydrogen collect on cathode and oxygen collect on anode?
-
Give three applications of chemical effect of current.
-
What precautions should be observed when doing electroplating?
-
Iron is a strong metal used for making bridges. Can we do electroplating on it to protect is from corrosion? Why?
-
Which properties of chromium make it useful for electroplating on iron? Why we cannot make the whole article with chromium?
-
Give some uses of LED. How should LED be connected?
-
What is the difference between current flowing through metals and current flowing through liquids?
Answer:
-
Bridges and automobiles are prevented from rusting by plating them with zinc.
-
LEDs require very little energy and glow even when a small current is passing through the circuit.
-
LED is a device in which the two terminals inside the glass bulb are connected together with a metallic point of Galluim Arsenide.
-
During electroplating, the silver metal from the anode is deposited on the cathode. Therefore, it becomes thin.
-
We can test the presence of the small current by using a LED instead of the bulb.
-
When electric current is passed through copper sulphate solution, the copper sulphate breaks up into copper and sulphate ions. The free copper gets drawn to the plate connected to the negative terminal of the battery and gets deposited on that plate. From the other plate an equal amount of copper gets dissolved in the solution. The loss of copper from solution is compensated and the process goes on.
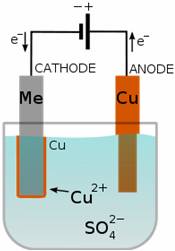
-
When electric current is passed in water hydrogen ions (OH+) being positive ions move towards cathode (negative terminal) and get collected over it. Hydroxyl ions (OH–) being negative ions move towards the anode (positive terminal) and oxygen is collected on it.
-
(a) Electroplating.
(b) Refining of impure metals.
(c) Refining of metal are for obtaining pure metal.
-
(a) The articles to be plated should be cleaned thoroughly to remove dust, grease or any deposit on it.
(b) Only direct current should be used.
(c) The temperature around the electroplating equipment should be optimum.
-
We prefer not to do electroplating on iron used for bridges because it is very costly. Instead the iron can be galvanized or painted.
-
Chromium has a good shine, it resists scratches and is corrosion resistant. We cannot make the whole article from it because it is a very costly metal.
-
(a) as indicators in electrical appliances.
(b) as a point source of light in laser beam torches.
(c) LEDs emitting white light can be used instead of bulbs.
The longer lead (leg) from the LED should be connected to the positive terminal of the battery and the shorter lead to the negative terminal .of the battery.
-
In metals electric current is conducted by flow of electrons. In liquids, the movement of charged particles (ions) carries current from anode to cathode terminals. Electrolytes conduct current at a slow rate than metals.
