LAWS OF CHEMICAL COMBINATION
Law of Conservation of Mass:
Example:
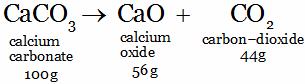
Question:
Sodium carbonate reacts with ethanoic acid to form sodium ethanoate, carbon dioxide and water. In an experiment, 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid to form 8.2 g of sodium ethanoate, 2.2 g of carbon dioxide and 0.9 g of water. Show that this data verifies the law of conservation of mass.
Sol:
All the we have to do in this problem is to calculate the mass of reactants and products separately, and then compare the two. If the two masses are equal, then the law of conservation of mass gets verified. The given reaction can be written as:
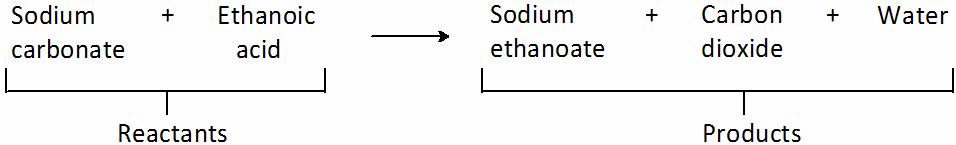
(i) Sodium carbonate and ethanoic acid are reactants.
So, Mass of reactants
= Mass of sodium carbonate
+ Mass of ethanoic acid
= 5.3 + 6
= 11.3 g
(ii) Sodium ethanoate, carbon dioxide and water are products.
So, Mass of products
= Mass of sodium ethanoate
+ Mass of carbon dioxide
+ Mass of water
= 8.2 + 2.2 + 0.9
= 11.3 g
We find that the mass of reactants is 11.3 g and the mass of products is also 11.3 g. Since the mass of products is equal to the mass of reactants, the given data verifies the law of conservation of mass.
Law of Constant Proportions:
“In a chemical substance the elements are always present in definite proportion by mass”.
For example:
Similarly:
Question:
In an experiment, 1,288 g of copper oxide was obtained from 1.03 g of copper. In another experiment, 3.672 of copper oxide on reduction gave 2.938 g of copper. Show that these figures verify the law of constant proportions.
Sol:
In order to solve this problem we have to calculate the ratio (or proportion) of copper and oxygen in the two samples of copper oxide compound. Now:
(a) In the first experiment: Mass of cooper = 1.03 g
And, Mass of copper oxide = 1.288 g … (1)
So, Mass of oxygen = Mass of copper oxide – Mass of copper
= 1.288 – 1.03
= 0.258 g … (2)
Now, in the first sample of copper oxide compound:
Mass of copper :
Mass of oxygen = 1.03 : 0.258
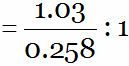
= 3.99 : 1
= 4 : 1 … (3)
(b) In the second experiment:
Mass of copper = 2.938 g … (4)
And, Mass of copper oxide = 3.672 g
So, Mass of oxygen = Mass of copper oxide – Mass of copper
= 3.672 – 2.938
= 0.734 g … (5)
Now, in the second sample of copper oxide compound:
Mass of copper : Mass of oxygen = 2.938 : 0.734
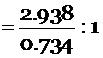
= 4 : 1 … (6)
From the above calculations we can see that the ratio (or proportion) of copper and oxygen elements in the two samples of copper oxide compound is the same 4 : 1. So, the given figures verify the law of constant proportions.
Question:
Hydrogen and oxygen combine in the ratio of 1: 8 by mass to from water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
Sol:
Here we have been given that hydrogen and oxygen always combine in the fixed ratio of 1 : 8 by mass. This means that:
1 g of hydrogen gas requires = 8 g of oxygen gas
So, 3 g of hydrogen gas requires = 8 × 3 g of oxygen gas
= 24 g of oxygen gas
Thus, 24 grams of oxygen gas would be required to react completely with 3 grams of hydrogen gas.
Question:
When 3 g of carbon is burnt in 8 g of oxygen, 11 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3 g of carbon burnt in 50 g of oxygen? Which law of chemical combination will govern your answer?
Sol:
Our answer will be governed by the law of constant proportions. Now, since carbon and oxygen, combine in the fixed proportion of 3 : 8 by mass to produce 11 g of carbon dioxide, therefore, the same mass of carbon dioxide (11 g) will be obtained even if we burn 3 g of carbon in 50 g of oxygen. The extra oxygen (50 – 8 = 42 g oxygen) will remain unreacted.
Question:
A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by mass.
Sol:
And, Mass of compound = 0.24 g
So, Percentage of boron (in compound)
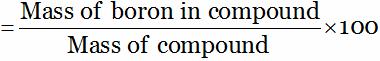
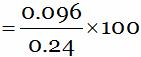
= 40 … (1)
And, Mass of compound = 0.24 g
So, Percentage of oxygen (in compound)
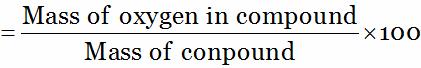
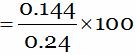
= 60 …(2)
Thus, the percentage composition of the compound is: Boron = 40 %: Oxygen = 60%.
Questions:
Answers: