CHEMICAL FORMULAE
-
The chemical formula of a compound is its symbolic representation.
-
It gives information about the number of atoms in its one molecule.
Chemical Formulae of Elements:
-
The chemical formula of an element is represented by writing the symbol of that element, and indicating the number of atoms in one molecule of it by a subscript on the right hand side of the symbol.
-
The number of atoms present in one molecule of an element is known as its atomicity.
-
A number of elements, such as helium and neon, exist as single atom. They are called monatomic.
-
The molecules of hydrogen, nitrogen, oxygen, chlorine, bromine, etc., contain two atoms of the element, respectively, and thus, their atomicity is two. Such molecules are said to be diatomic (two atoms in a molecule).
-
A molecule of phosphorus has four phosphorus atoms.
-
Thus, a molecule of phosphorus is tetraatomic and its atomicity is 4.
-
A molecule of nitrogen has two atoms of Nitrogen in it. Its molecule can be represented as N2.
-
Symbol of the element → N2 subscript showing atomicity of that element
Chemical Formula of a Compound:
-
In a molecule, the atoms of various elements are always present in whole numbers.
-
The molecular formula (or chemical formula) of a compound is written as follows:
-
The symbols of the constituent elements present in a molecule are written side by side.
For example:
-
If we have to write the formula of Hydrogen chloride (also called hydrochloric acid); the symbols of the constituent elements would be H and CI.
-
The number of atoms of various elements are represented as subscript to each respective symbol.
-
Since one molecule of hydrogen chloride contains one atom of hydrogen and one atom of chlorine, so the molecular formula of hydrogen chloride will be H1Cl1 or HCl (subscript 1 is not written).
-
Similarly, a molecule of water contains two hydrogen atoms and one oxygen atom so the formula of water will be H2O.
-
A molecule of aluminum oxide contains two aluminum atoms and three oxygen atoms so the formula of aluminium oxide will be Al2O3.
-
To represent more than one molecule of a substance (compound/element) or atom of an element, the number is put as a prefix to the formula or the symbol.
For example:
-
2CO2 means two molecules of carbon dioxide or 4HCl represents four molecules of hydrogen chloride.
-
Similarly, 2He stands for two atoms of helium (since helium is monatomic).
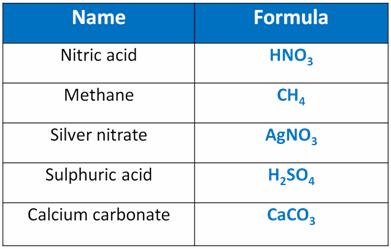
Chemical Formulae of Some Compounds:
Significance of a Chemical Formula:
-
The chemical formula of a compound indicates:
-
The different elements contained in a molecule of a compound.
-
The number of atoms of each element in one molecule of the substance.
