CHEMICAL FORMULAE
Formulae of Elements:
For example:
H2 2 atoms of Hydrogen in one molecule
N2 2 atoms of Nitrogen in one molecule
O2 2 atoms of Oxygen in one molecule
Cl2 2 atoms of Chlorine in one molecule
Br2 2 atoms of Bromine in one molecule
I2 2 atoms of Iodine in one molecule
P4 4 atoms of Phosphorous in one molecule
P8 8 atoms of Phosphorous in one molecule
Formulae of compounds:
For example:
H2 2 atoms of Hydrogen in one molecule
N2 2 atoms of Nitrogen in one molecule
O2 2 atoms of Oxygen in one molecule
Cl2 2 atoms of Chlorine in one molecule
Br2 2 atoms of Bromine in one molecule
I2 2 atoms of Iodine in one molecule
P4 4 atoms of Phosphorous in one molecule
P8 8 atoms of Phosphorous in one molecule
Writing chemical formula of a molecular compound:
Step 1:
Write the symbols of the elements present in the compound.
Example:
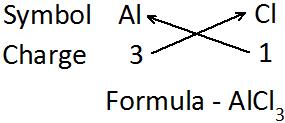
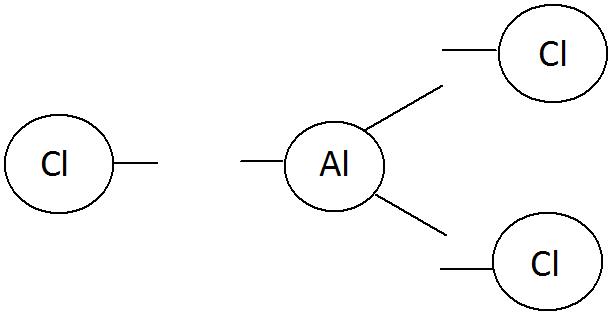
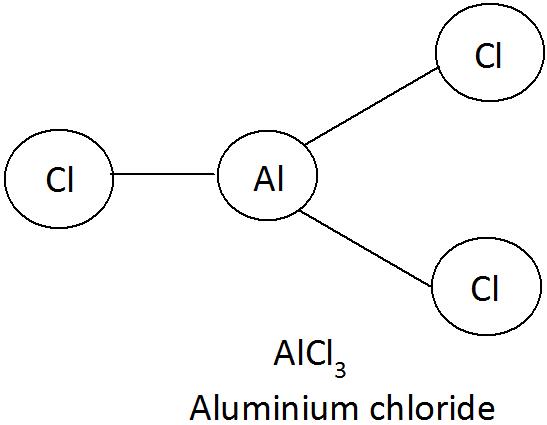
Aluminium chloride
Aluminium [Al] Chlorine [Cl]
Step 2:
Write the valency of each element.
Al valency 3 and Cl valency 1 (Cross placement of valency)