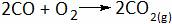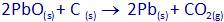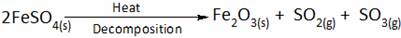Chemical reaction and equations worksheet-3
Multiple Choice Questions:
-
An aqueous solution is formed using _____ as solvent.
(A) Hydrogen chloride (B) Sulphuric acid
(C) Alcohol (D) Water
(E) Sodium hydroxide
-
What is the chemical formula of quick lime or lime?
(A) CaCO3 (B) CaO (C) Ca(OH)2 (D) CoCl2
(E) None
-
What is precipitation reaction?
(A) A reaction in which oxidation takes place
(B) A reaction in which an insoluble substance is formed
(C) A reaction in which reactants do not react
(D) A reaction in which a soluble substance is formed
(E) None
-
Calcium hydroxide solution when applied to the walls reacts with a gas present in air forming a substance which gives shiny texture to the wall. What is the gas and the product?
(A) Nitrogen, calcium nitride
(B) Hydrogen, Calcium hydride
(C) Oxygen, calcium oxide
(D) Carbon-di-oxide, Calcium carbonate
-
What is the chemical formula of Calcium fluoride?
(A) CaF (B) Ca(HF)2 (C) CaF2 (D) None
-
Rusting is the result of
(A) Oxidation (B) Combustion (C) Reduction (D) Precipitation
(E) Combination
-
What is the product formed when ferrous sulphate is heated strongly?
(A) Fe2O3, SO2, SO3 (B) FeS,SO2,SO3
(C) FeO, SO2 (D) Fe3O4, SO2
(E) All the above
-
Which of the following is double displacement reaction?
(A) 
(B) 
(C) 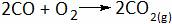
(D) 
-
Which of the statements about the reaction below are incorrect?
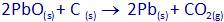
(i) Lead is getting reduced.
(ii) Carbon dioxide is getting oxidized.
(iii) Carbon is getting oxidized.
(iv) Lead oxide is getting reduced.
(a) i and ii (b) i and iii
(c) i, ii and iii (d) all
(A) (A) (B) (C) (C) (D) (D) (E)
-
Fe2O3 + 2Al → Al2O3 + 2Fe The given reaction is an example of
(A) Combination reaction
(B) Double displacement reaction
(C) Decomposition reaction
(D) Displacement reaction
Answer keys:
-
(D)
-
(B)
-
(B) Precipitate is an insoluble substance which separates out from the solution during a chemical reaction.
-
(D) Calcium hydroxide reacts with atmospheric carbon-di-oxide forming calcium carbonate which gives shiny texture to the wall.
-
(C) The valency of calcium is 2 and fluorine is 1 so the formula of calcium fluoride is CaF2
-
(A) Iron reacts with oxygen and moisture of atmosphere and forms iron oxide (Fe2O3.xH2O)
-
(A)
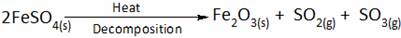
-
(D) In a double displacement reaction the reactants react by exchange of ions.
-
(A) Lead oxide is reduced and carbon is oxidized as carbon di-oxide is formed.
-
(D) Aluminum being more reactive than iron displaces it from its oxide. This reaction is used for repairing railway tracks (Gold smith’s Aluminothermite process)