CHEMICAL EQUATION
Thus a chemical equation is a short form that is used to describe a chemical reaction.
Example:
Carbon burns in air to form carbon dioxide gas releasing heat in the process.
Carbon is the reactant. Carbon dioxide is the product. Heat energy is released in the conversion.
Chemical equation:
C + O2 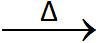 CO2 + Heat released
CO2 + Heat released
Reaction of Hydrogen with Oxygen forming water. 2H2 + O2 → 2H2O
Potassium chlorate undergoing decomposition on heating to form potassium chloride and oxygen.
2KCIO3 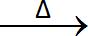 2KCI + 3O2
2KCI + 3O2
Reaction of Silver nitrate with hydrogen chloride forming silver chloride and nitric acid.
AgNO3 + HCl → AgCl + HNO3
Zinc reacts with dilute hydrochloric acid to form zinc chloride and liberates hydrogen.
Zn + 2HCl → ZnCl2 H2
Balancing a Chemical Equation:
According to Law of mass action:
Balancing a chemical equation:
Step 1:
Step 2:
Note:
Example:
The chemical reaction between hydrogen and oxygen forming water.
The unbalanced equation is written as
H2 + O2 → H2O
|
Elements/ compounds |
Reactant side |
Product side |
|
Number of Hydrogen atoms |
2 |
2 |
|
Number of Oxygen atoms |
2 |
1 |
H2 + O2 → 2H2O
Now:
|
Elements/ compounds |
Reactant side |
Product side |
|
Number of Hydrogen atoms |
2 |
4 |
|
Number of Oxygen atoms |
2 |
2 |
2H2 + O2 → 2H2O
|
Elements/ compounds |
Reactant side |
Product side |
|
Number of Hydrogen atoms |
4 |
4 |
|
Number of Oxygen atoms |
2 |
2 |
Equation is balanced.
Li2O + H2O → LiOH
|
Elements or compounds |
Reactant side |
Product side |
|
Number of atoms of Lithium |
2 |
1 |
|
Number of atoms of Hydrogen |
2 |
1 |
|
Number of atoms of oxygen |
2 |
1 |
Put 2 in front of LiOH.
Li2O + H2O → 2LiOH
|
Elements or compounds |
Reactant side |
Product side |
|
Number of atoms of Lithium |
2 |
2 |
|
Number of atoms of Hydrogen |
2 |
2 |
|
Number of atoms of oxygen |
2 |
2 |
The chemical equation is balanced.
FeCl3 + NaOH → Fe(OH)3 + NaCl
|
Elements or compounds |
Reactant side |
Product side |
|
Number of atoms of iron |
1 |
1 |
|
Number of atoms of chlorine |
3 |
1 |
|
Number of atoms of oxygen |
1 |
3 |
|
Number of atoms of hydrogen |
1 |
3 |
|
Number of atoms of sodium |
1 |
1 |
FeCl3 + 3NaOH → Fe(OH)3 + NaCl
|
Elements or compounds |
Reactant side |
Product side |
|
Number of atoms of iron |
1 |
1 |
|
Number of atoms of chlorine |
3 |
1 |
|
Number of atoms of oxygen |
3 |
3 |
|
Number of atoms of hydrogen |
3 |
3 |
|
Number of atoms of sodium |
3 |
1 |
FeCl3 + 3NaOH → Fe(OH)3 + 3NaCl
|
Elements or compounds |
Reactant side |
Product side |
|
Number of atoms of iron |
1 |
1 |
|
Number of atoms of chlorine |
3 |
3 |
|
Number of atoms of oxygen |
3 |
3 |
|
Number of atoms of hydrogen |
3 |
3 |
|
Number of atoms of sodium |
3 |
3 |