Materials: Metals and non-metals Worksheet-2
A. Sulphuric acid B. Sulphur trioxide
C. Sulphurous acid D. Sulphur dioxide
A. Sulphur B. Sulphur trioxide
C. Sulphuric acid D. Sulphurous acid
A. NaHSO3 B. Na2SO3 C. Na2SO4 D. Na2S
A. Red B. Blue C. Yellow D. Green
A. Basic B. Acidic
C. Neutral D. All of these
A. Water B. Alcohol C. Kerosene D. Ether
A. Aluminium B. Potassium
C. Calcium D. Magnesium
A. Sodium B. Phosphorous
C. Calcium D. Uranium
A. CuSO4 B. CuS C. Cu2S D. Cu2S2O3
A. Oxygen B. Nitrogen
C. Hydrogen D. Carbon dioxide
Answer:
Explanation: S + O2 → SO2
When sulphur is burnt in air or oxygen sulphur dioxide gas is formed.
Explanation: Sulphur dioxide dissolves in water producing sulphurous acid.
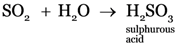
Explanation: NaHSO3 is sodium bisulphite, Na2SO3 is sodium sulphite, Na2SO4 is sodium sulphate, Na2S is sodium sulphide
Explanation: The aqueous solution of carbon dioxide is acidic in nature hence it will change the colour of litmus paper to red.
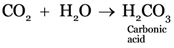
Acids turn blue litmus red.
Explanation: Sodium is highly reactive and on exposure to air its surface gets tarnished hence it is kept in kerosene.
Explanation: Potassium reacts vigorously with oxygen and water forming its oxide and hydroxide. Large amount of energy is released in the reaction
Explanation: Phosphorous readily catches fire in air giving dense fumes of Phosphorous pentoxide hence is kept in water.
Explanation: CuSO4 is copper sulphate, CuS is cupric sulphide, Cu2S is cuprous sulphide, Cu2S2O3 is cupric thiosulphate
Explanation: 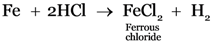
Hydrogen gas is released when metals react with acids.