Structure of the atom Worksheet-6
-
What are canal rays?
-
If an atom contains one electron and one proton, will it carry any charge or not?
-
On the basis of Thomsons model of an atom explain how the atom is neutral as a whole.
-
On the basis of Rutherford's model of an atom, which sub-atomic particle is present in the nucleus of an atom?
-
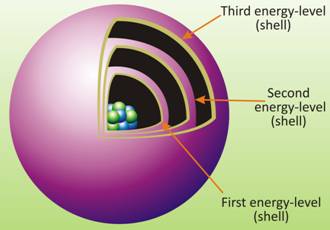
Draw a sketch of Bohr's model of an atom with three shells.
-
What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
-
Helium atom has atomic mass of 4u and has two protons in its nucleus. How many neutrons does it have?
-
What is the distribution of electrons in oxygen and chlorine atoms?
-
If the K and L shells of an atom are full, then what would be the number of electrons in the atom?
-
If the number of electrons in an atom is 8 and the number of protons is also 8, then :
(i) What would be the atomic number of the atom?
(ii) What is the charge on the atom?
Answer:
-
Initially anode rays were called canal rays. These rays consist of positively charged particles. These rays are directed towards cathode. However, they do not originate from the anode.
-
The atom will be neutral i.e. it will not carry any charge as it contains one proton and one electron. The charges neutralize each other.
-
According to Thomson's Model of an atom, an atom may be regarded as a positively charged sphere in which protons are present. The negatively charged electrons are studded or embedded in the sphere. Equal number of protons and electrons are present in the atom. The negative charges due to electrons balance the positive charges due to protons hence an atom is neutral as a whole.
-
According to Rutherford’s model of atom the nucleus of atom is positively charged. All the protons in an atom are therefore, present in the nucleus.
-
-
If the metal is a heavy one like gold (e.g. silver, platinum etc.) similar results will be obtained. However if the metal is quite light (e.g. sodium, magnesium etc.), it is just possible that the fast moving and massive α -particles (4u mass) may push the nucleus aside and pass through with slight deviations only.
-
Mass number of helium is equal to its atomic mass but has no units.
Mass number (A) of helium = 4
No. of protons in the nucleus = 2
Atomic number (Z) of the element = 2
No. of neutrons (n) = A – Z = 4 – 2 = 2.
-
Oxygen (Z = 8) : 2 (K-shell) ; 6(L-shell)
Chlorine (Z = 17) : 2 (K-shell) ; 8 (L-shell) ; 7 (M-shell)
-
Maximum no. of electrons in K-shell = 2
Maximum no. of electrons in L-shell = 8
Total no. of electrons in the atom = 2 + 8 = 10
-
(i) Atomic number (Z) of the atom = No. of protons
= No. of electrons = 8
(ii) There will be no charge on the atom. Actually, when the number of protons and electrons are the same, the atom is neutral.
