FLAME
-
Flame is the zone of combustion of a combustible substance. Substances which vapourise during burning produce flames.
-
Eg:- kerosene, wax,oils etc.

-
Substances which do not vapourise during burning do not produce flames.
-
Eg:- coal, charcoal etc.
-
The colour of the flame depends on the temperature, amount of air available, and the nature of the substance burning. Hydrocarbons burn with blue or yellow flame depending upon the amount of oxygen available.

Change in the colour of a Bunsen burner flame with increasing oxygen supply:
-
A yellow flame is also called a luminous flame, as it emits a lot of light. A luminous flame is generally observed when there is insufficient oxygen (i.e., incomplete combustion). Its temperature is lower than that of a blue flame and it leaves behind black soot and other residue.
-
A blue flame is also called a non-luminous flame as it emits very little light. A blue flame is generally observed when there is adequate amount of oxygen available (i.e., complete combustion). This type of flame leaves behind no residue.
-
A flame does not show uniform colour .There are different zones in a flame.
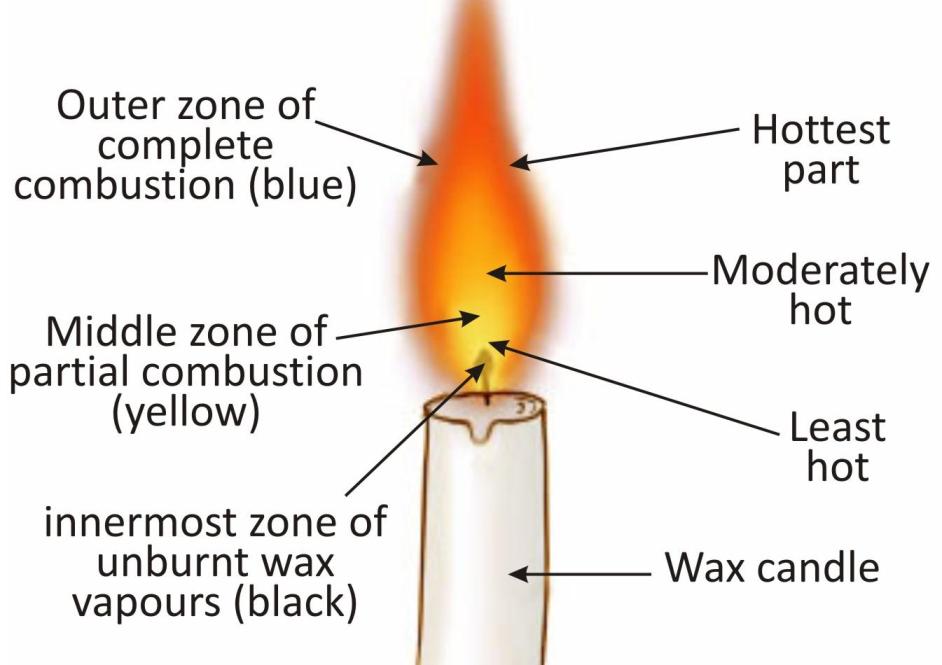
Zones of a Candle Flame:

-
A candle flame can be divided into three zones, depending on the amount of oxygen available.
-
The outer zone (blue) is the hottest part of the flame. In this zone, the wax vapours have enough oxygen to burn completely (producing carbon dioxide and water).This zone emits very little light.
-
The middle zone (yellow) is less hot than the outer zone. Here, incomplete combustion of wax vapours (due to low oxygen) produces carbon particles (which glow, giving the zone its yellow colour) and carbon monoxide. This zone emits the most light.
-
The inner zone (black) is the coolest part of the flame. In this zone, the wax vapours remain unburned as no oxygen is available. This zone is completely dark and emits no light.
Combustion of a Wax Candle:
-
If you observe a candle flame closely, you will notice the following.
-
The wick burns and it stands in a pool of liquid wax.
-
There is a small portion of unburnt wick between the flame and the liquid wax.
-
The liquid wax is trapped in a 'cup' of solid wax.
-
The liquid or solid wax never catches fire.
-
We can see that the wick is burning. But it cannot be the only substance that is burning as the candle gets smaller as it burns. The wax vapours also burn.
-
If a lit match is brought a little above a candle wick immediately after the candle has been blown out, the flame from the match jumps the gap and reignites the wick. This happens because the wax vapours rise from the wick immediately after the candle is blown out and the burning match reignites them.
Conclusion:
-
It is only the wax vapours that burn. Neither liquid wax nor solid wax burns.
-
When a candle wick is lit, the heat produced from the flame melts the wax.
-
The wick soaks or absorbs the molten wax.
-
The heat of the flame vaporizes the molten wax in the wick.
-
The wax vapours burn in the flame. This process continues till the entire wax is consumed or the candle is extinguished.



