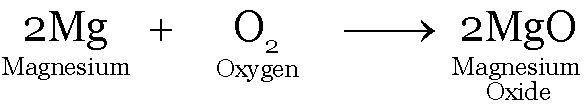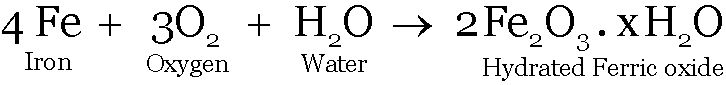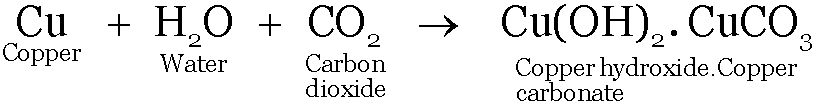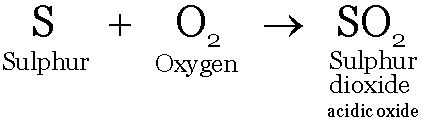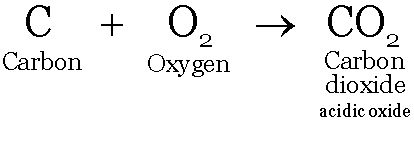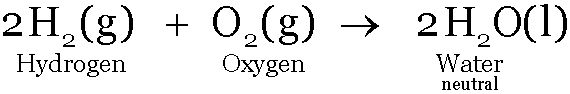REACTION OF METALS AND NON METALS WITH OXYGEN
Reaction of metals with oxygen:
-
Metals react with oxygen producing respective oxides.
-
Metals like sodium (Na) and potassium (K) are some of the most reactive metals.
-
Sodium metal reacts with the oxygen of the air at room temperature to form sodium oxide. The reaction is vigorous hence sodium is stored under kerosene to prevent its reaction with oxygen, moisture and carbon dioxide.
-
Rusting of iron, burning of Mg ribbon in air
-
In both processes oxides are formed.
Reaction:
-
Mg ribbon burns in air on heating giving a basic oxide of magnesium oxide.
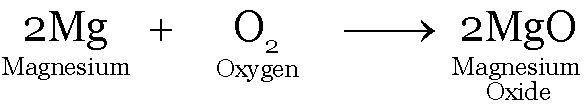
Corrosion:
-
Iron metal reacts with air and moisture forming hydrated iron oxide.
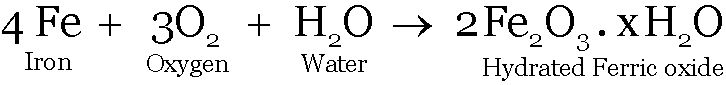

Rust on Iron Object
-
When a copper vessel is exposed to moist air it acquires a dull green coating. Green material formed is a mixture of Copper hydroxide & copper carbonate.
The reaction taking place on the surface of copper is-
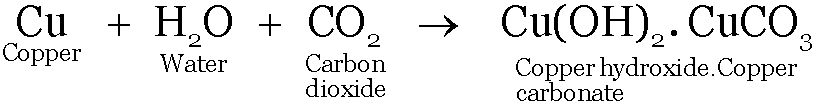
Properties of Metal Oxides
-
In general, metallic oxides are basic in nature.
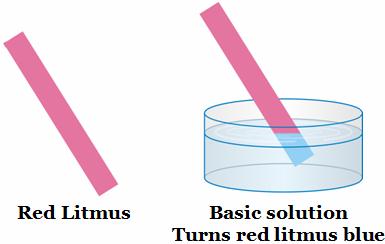
Acidic or basic nature of any substance can be tested by litmus paper.
-
In acidic solution, blue litmus turns to red.
-
In basic solution, Red litmus turns to blue chemical reactions of non metals
Magnesium Oxide
-
The ash obtained on burning Mg ribbon is dissolved in water.
-
The solution obtained turns red litmus blue.
Iron oxide
Iron oxide is also basic in nature. Suspension of rust also changes red litmus blue.

Reaction of Nonmetals with Oxygen:
-
Non metals react with oxygen forming non metal oxides. These oxides are acidic or neutral in nature.
Example:
-
Carbon dioxide and Sulphur dioxide are acidic in nature; water is a neutral oxide of hydrogen.
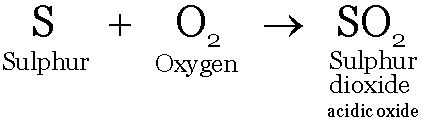
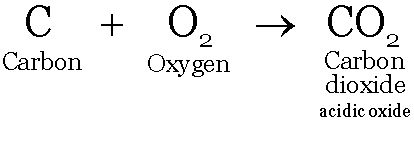
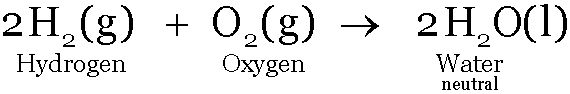
-
These oxides dissolve dissolve in water to form acids.


-
These solutions turn blue litmus red.