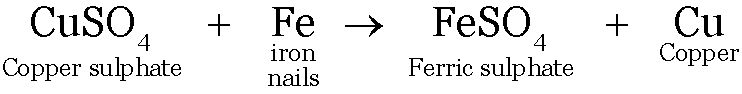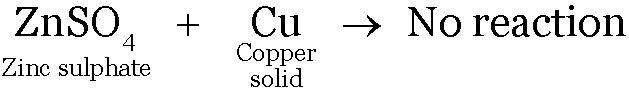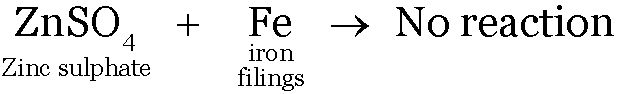REACTION OF METALS AND NON METALS WITH BASES
-
Metals react with NaOH producing hydrogen gas.
-
Nonmetals undergo complex reactions with NaOH.
Displacement Reaction:
-
When a more reactive metal is put in the salt solution of a less reactive metal, the more reactive metal displaces the less reactive metal from its solution.
-
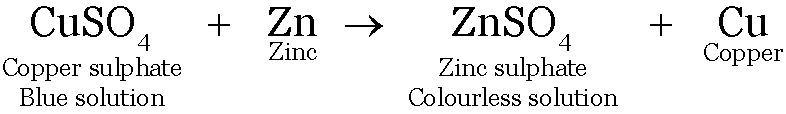
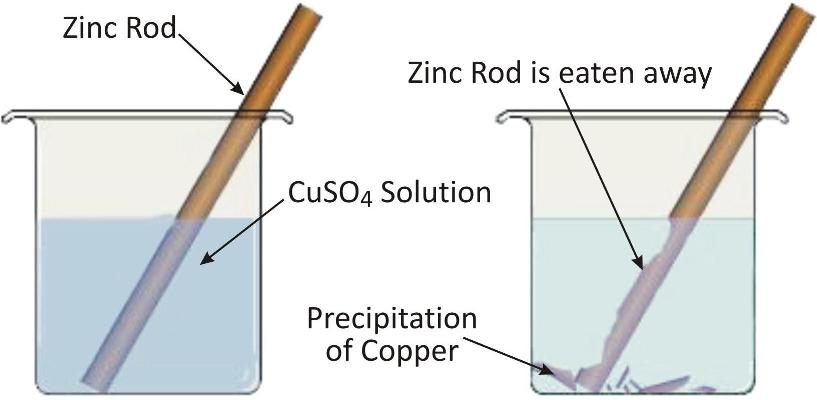
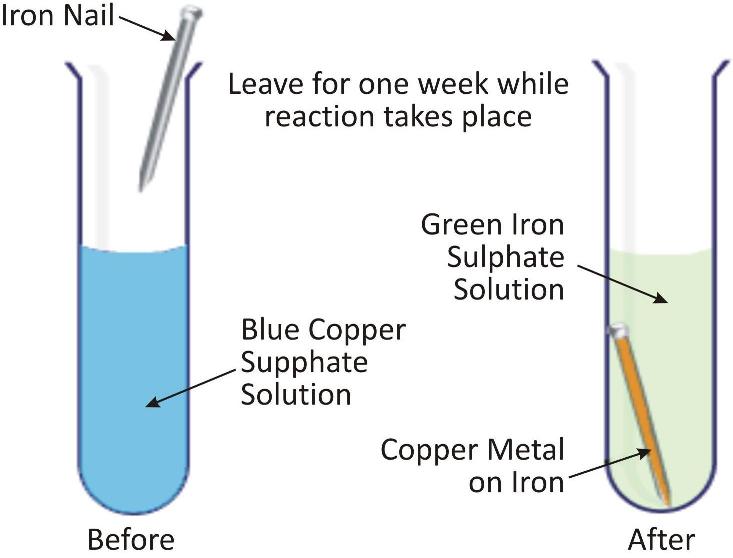
Eg: When a strip of Zn is put in CuSO4 solution. The blue colour of CuSO4 solution fades due to formation of colourless
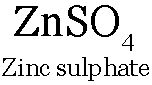 and
and 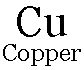 metal is deposited on the Zn strip.
metal is deposited on the Zn strip.
-
Zinc being more reactive displaces copper from copper sulphate solution.
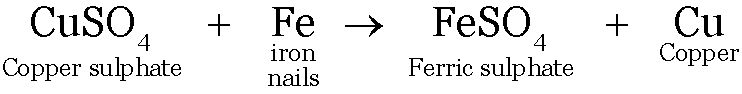
-
Iron being more reactive displaces copper from copper sulphate solution
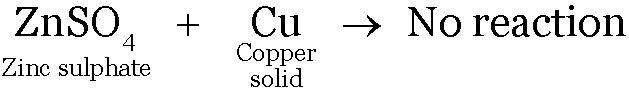
-
Copper being less reactive than zinc will not displace Zinc from the solution.
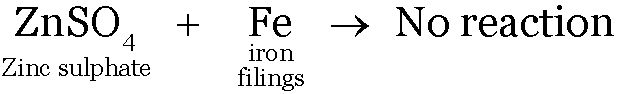
-
Iron being less reactive than Zinc will not displace Zinc from the solution.
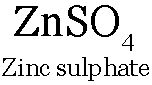 and
and  metal is deposited on the Zn strip.
metal is deposited on the Zn strip.