INTRODUCTION
-
We see many things and changes around us. These things are made of different types of materials. Every day we come across many changes in our surroundings too. These changes may involve one or more substances.
For example:
-
Cooking of vegetables, setting curd from milk and even souring of milk is a change.
-
Stretched rubber band, paper tore into pieces, Chalk crushed into powder, changing of water into steam are also changes.
-
Properties such as shape, size, colour and state of a substance are called physical properties.
-
A process in which substance undergoes change in its physical properties is known as physical change. In such a change no new substance is formed.
Chemical change:
-
A change in which a new substance is formed is a chemical change. A chemical change is also called chemical reaction.
-
In order to understand a chemical reaction we will first study about chemical substance, its classification and also about chemical formulae.
Chemical substance:
-
A form of matter that has constant chemical composition and characteristic properties, which cannot be separated by physical methods of separation like sieving, sedimentation etc. are called chemical substances.
-
Matter of definite composition and properties.
-
A common example of chemical substance is pure water it has its characteristic properties, similar chemical composition whether its tap water or water of any river.
-
Salt water is not a chemical substance it is a mixture of two substances- sodium chloride and water, its composition is hence not fixed.
-
Chemical substances are made of atoms and molecules.
-
An atom is the smallest particle of a substance. Atoms may or may not exist independently.
-
Molecules are formed when two or more atoms join together and they can exist independently.
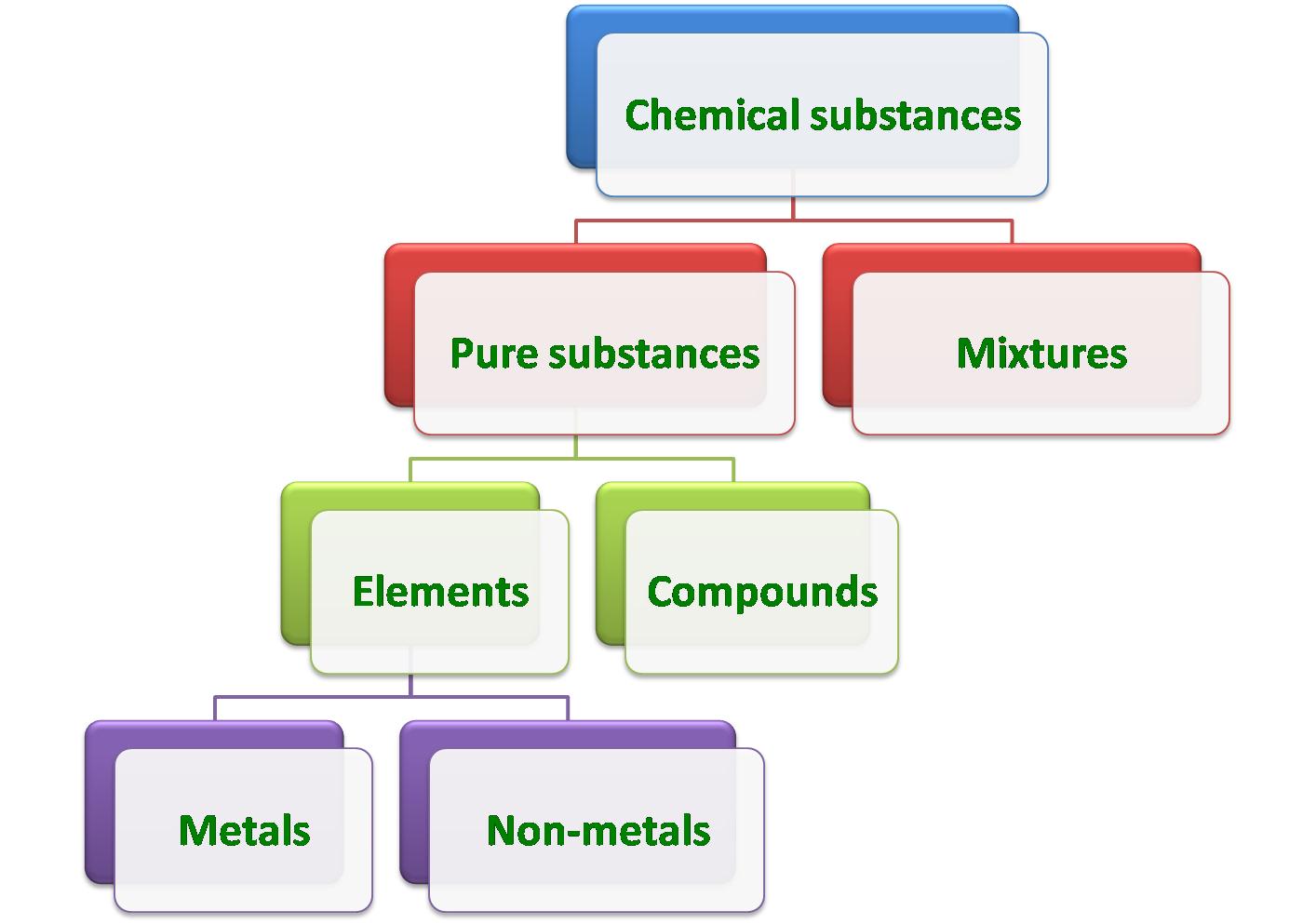
Chemical substances can be divided into two major groups: