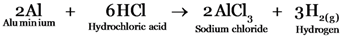Materials: Metals and non-metals Worksheet-7
-
What happens when Hydrochloric acid is poured on aluminium foils?
-
Sodium is dropped in water?
-
A copper spoon had fallen into a container containing dil. HCl. What would happen to it if it is dipped in it for four days?
-
Give reasons for the following :
(a) Metals are used for making bells.
(b) We can't use pure gold to make jewellery.
-
A metal ribbon burns in air with bright white light and forms a white powder.
Which metal is this?
Give the equation of the reaction taking place.
The metallic oxide formed would be acidic or basic in nature?
-
Give reason: Silver is used in making mirrors.
-
Give reason: Aluminium is used to make electrical wire.
-
Iron is used in construction of bridges and houses.
-
Graphite is used as an electrode in the dry cell.
-
Give reason: Iron sheets are galvanized before use.
Answer:
-
Aluminium will react with hydrochloric acid forming Aluminium chloride and liberating Hydrogen gas.
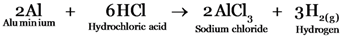
-
Sodium will react with water forming sodium hydroxide and hydrogen. This reaction is vigorous.
2Na + H2O → 2NaOH + H2(g) + Heat
-
In the presence of air copper dissolves in dil HCl and in the absence of air HCl has no effect on it. In the container due to absence of oxygen there will be no effect on the spoon.
2Cu + 4HCl → No reaction
2Cu + 4HCl + O2 → 2CuCl2 + 2H2O
-
(a) Metals are sonorous hence are used for making bells
(b) Pure gold is very soft hence it is not used for making jewellary.
-
Magnesium ribbon burns in air with bright white light forming Magnesium oxide.
2Mg + O2 → 2MgO
The oxide formed is basic in nature.
-
Silver is used in making mirrors because it has:
(a) Low density so weight is comparatively lesser than other white coloured metal.
(b) Silver is used in glass considering its strength, non corrosive, and reflection property.
-
Aluminium is good conductor of electricity hence it is used for making wires.
-
Iron has high tensile strength, is malleable and ductile hence it is used in construction of bridges and houses.
-
Graphite is good conductor of electricity and its reactivity is also less hence it is used as electrode in the dry cell.
-
The phenomenon of coating iron with zinc is called galvanization. Galvanization is done to prevent corrosion.