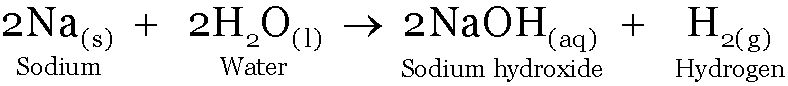REACTION OF METALS AND NON METALS WITH WATER
Reaction of metals with water
-
Metals react with water forming hydroxides.
Example:
-
Sodium reacts with water forming sodium hydroxide and Hydrogen, heat is released.
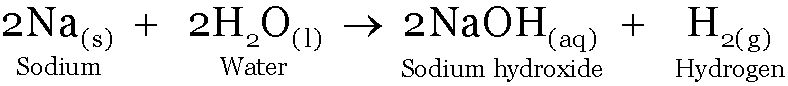
-
Potassium reacts with water producing potassium hydroxide and Hydrogen gas. The reactivity of potassium with water is greater than that of sodium. The reaction is so violent that the hydrogen gas evolved catches fire.
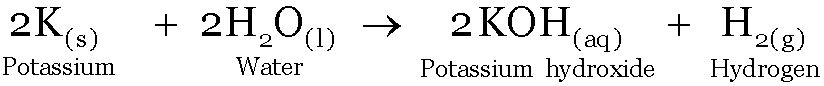

Reaction of Potassium with water
-
Non-metals generally do not react with water. Though they may be very reactive in air
Example:
-
Phosphorous is highly reactive, and catches fire if exposed to air. To prevent its contact with air it is always stored under water.