DISCOVERY OF PROTON
-
The existence of protons in the atoms was shown by E. Goldstein.
-
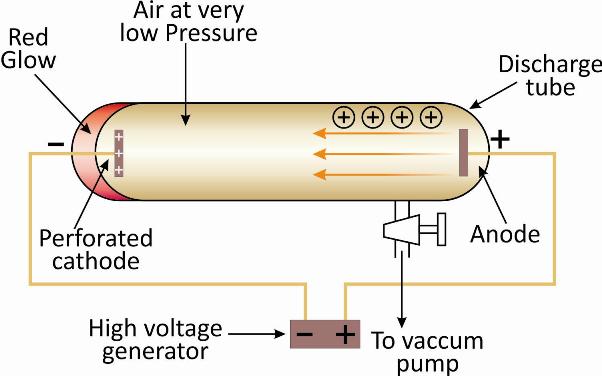
E. Goldstein, a German scientist repeated the Thomson’s experiment by modifying the discharge tube.
-
He used a perforated cathode and it was placed in the centre of the tube.
-
Hydrogen gas was enclosed in the discharge tube and the experiment was performed a was with cathode ray tube.
-
He found that rays were emitted in the tube which passed through the perforations in the cathode and moved away from the anode.
-
E. Goldstein called these rays canal rays.
-
The rays were later named as anode rays as they were directed away from the anode.
The origin of anode rays:
-
The discharge tube contains Hydrogen gas in it.
-
Under high voltage, these molecules break into atoms of hydrogen.
-
The high speed electrons which constitute the cathode rays collide with hydrogen atoms.
-
As a result, electrons are knocked out of these atoms leaving behind positively charged residues. These positively charged residues constitute the anode rays and move through the perforations of cathode.
-
Hydrogen gas was enclosed in the discharge tube, so these positively charged residues were called protons. Proton arises from the word protium which represents hydrogen atom.
-
When experiment was performed with some other gas the positive residues were different and were actually aggregate or multiple of protons.
-
A proton is usually represented by the symbol p*.
Characteristics of anode rays:
-
Anode rays travel in straight line.
-
Anode rays consist of positively charged particles known as protons.
-
The nature of anode rays depends upon the gas enclosed in the discharge tube.
-
Anode rays are deflected by electric field and magnetic field in a direction opposite to the cathode rays.
-
The specific charge (e/m ratio) is not the same for all the gases enclosed in the discharge tube.
Characteristics of a Proton:
-
Mass of a Proton:
-
The relative mass of a proton is 1 u.
-
The mass of proton is 1.673 × 10–27 kg.
-
Charge of a Proton:
-
The charge of a proton is equal and opposite to the charge of an electron i.e. 1.602 × 10–19 Coulomb
-
Relative charge of a proton is +1.
