TYPES OF BONDS
-
Ionic bond
-
Covalent bond
-
Coordinate bond
Ionic Bond:
-
An atom forms a bond to complete its octet i.e. attain 8 electrons in its outer most shell or valence shell.
-
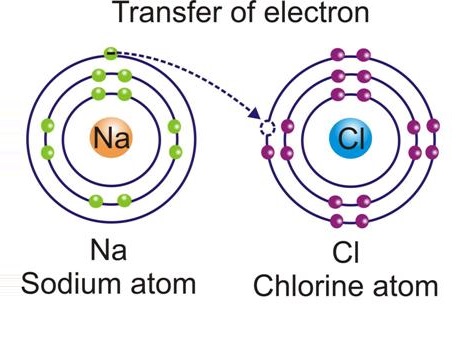
An ionic bond is formed between a metal and a non-metal. A metal atom loses electrons and a non-metal atom gains electrons for completion of their octet forming cations and anions respectively.
-
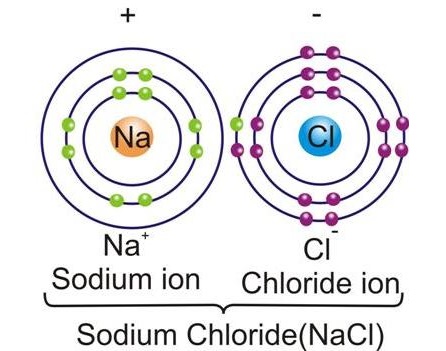
The electrostatic force of attraction between the positive and negative charged ions brings the particles together forming an ionic compound.
Sodium chloride Na+ + Cl– → NaCl
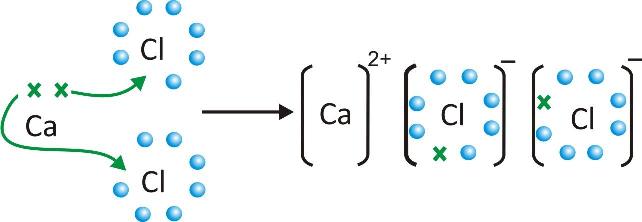
Formation of calcium chloride CaCl2
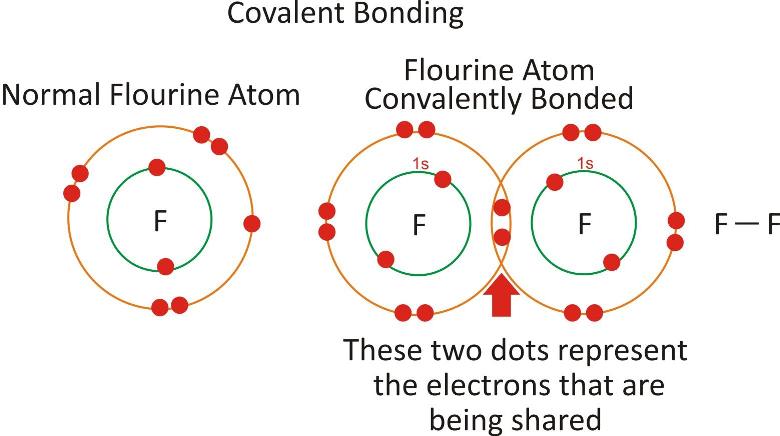
Covalent Bond:
-
The bond formed by mutual sharing of electrons between the bonding atoms which may be either same or different is called covalent bond. The compound formed is called covalent compound.
-
This pair of electron counts towards both the atoms and helps them to achieve them the configuration of nearest noble gas element.
-
The electrons involved in sharing are known as shared pair of electrons.
Single covalent bond:
-
It is formed by sharing of single electron by each of the participating atom.
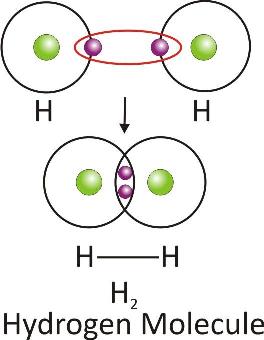
Hydrogen Molecule :
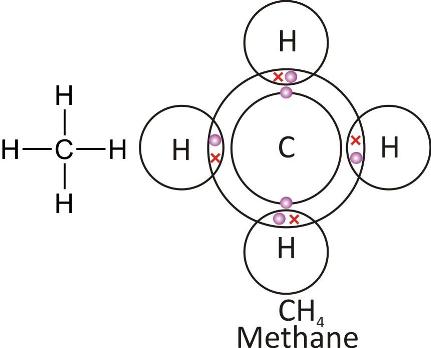
Methane molecule:
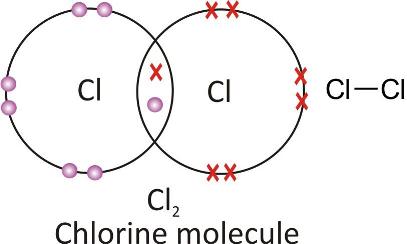
Chlorine molecule :
Water molecule:
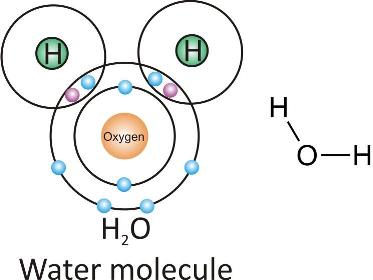
Ammonia molecule:
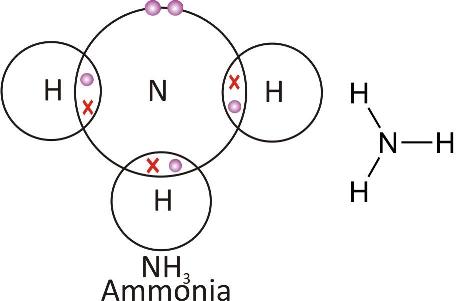
Double covalent bond:
-
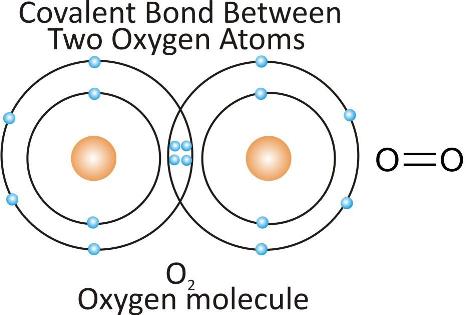
A double covalent bond is formed by sharing of a pair of electron by each of the participating atoms.
Oxygen molecule:
Carbon dioxide molecule:
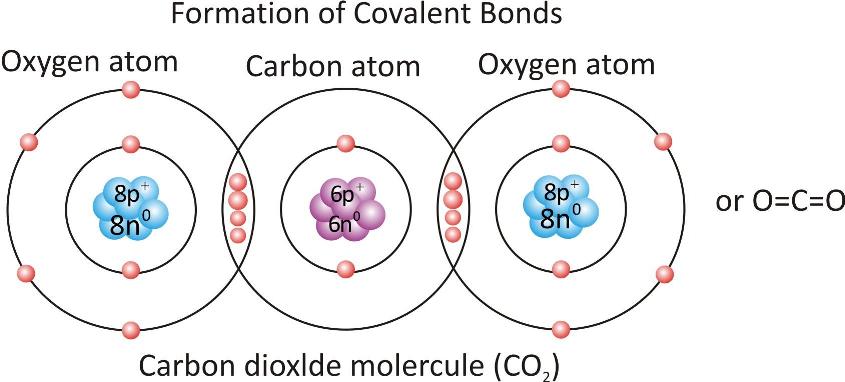
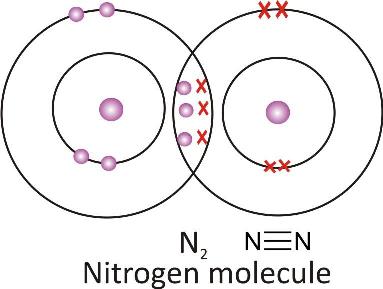
Formation of triple covalent bond:
-
A triple covalent bond is formed by sharing of three electrons by each of the participating atoms.
Nitrogen molecule: