ACIDIC SUBSTANCES
Acidic substances contain acids. Acids are of two types:
-
Mineral or laboratory acids
-
Organic acids
Acids can be strong like mineral acids or weak like organic acids.
Mineral Acids/Laboratory Acids:
-
An acid which is derived from one or more inorganic elements like Chlorine, Sulphur, Nitrogen, Phosphorous etc is called inorganic acid.
-
Hydrochloric acid (HCl), sulphuric acid (H2SO4), nitric acid (HNO3), etc. are some examples of mineral acids that are used in the laboratory.

-
Mineral acids such as nitric acid and sulphuric acid can destroy human tissues, clothes, paper, etc.
-
Thus, Acids should be handled carefully as they can cause burns upon contact with the skin.
Organic Acids:
-
Acids which occur naturally in animal and plant materials are called organic acids.
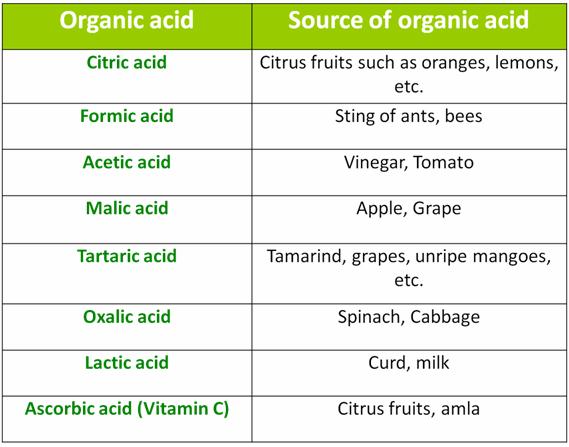
Some organic acids and their sources:
Properties of acids:
-
Some of the characteristic properties of acids are:
-
Acids have a sour taste and are corrosive in nature: Sour taste is one of the main characteristic of acids.
-
Acids also have the ability to corrode metals such as iron and aluminum. For this reason, acids are generally stored in glassware.
-
Acids are soluble in water.
-
Most acids dissolve in water either at room temperature or on heating to form a clear solution.
-
For example, vinegar is a 3-5% solution of acetic acid in water.
-
Hydrofluoric acid is the only known acid that attacks glass and is therefore stored in plastic bottles.
-
Since it dissolves glass it is also used to etch glass.
-
Hydrofluoric acid is highly toxic and can kill upon exposure.
-
Depending on the amount of water, acids can be either dilute or concentrated.
-
If the amount of water is more in an acid, it is called diluted acid and if the amount of water is less, it is called concentrated acid.
Uses of Acids:
1. Hydrochloric acid (HCl):
-
Dilute hydrochloric acid is used in various industries for removing deposits from the inner wall of boilers. This process is known as de-scaling.

-
Hydrochloric acid is also used for cleaning sinks and sanitary ware. Harpic is the brand name of a toilet bowl cleaner launched in England in the 1920s and now marketed by Reckitt Benckiser. Its active component is Hydrochloric acid.
-
It also used in the crystallization of common salt.
2. Sulphuric Acid (H2SO4):
-
Sulphuric acid is also called as king of chemicals as it is used in every chemical process in industries.
-
Due to its oily appearance, it is called as oil of vitriol.
Some of its important uses are as follows:
-
Sulphuric acid is used in car batteries.

-
It is used in the manufacture of paints, drugs, dyes, and artificial silk.
-
It is also used to produce fertilizers such as ammonium sulphate, super phosphate etc.
3. Nitric Acid (HNO3):
-
Nitric acid is used in the manufacture of explosives such as TNT (trinitrotoluene) and nitroglycerine.
-
A mixture of Nitric acid and Hydrochloric acid is used by goldsmiths for cleaning gold and silver ornaments.
-
It is also used for the production of fertilizers such as ammonium nitrate.
Uses of Organic acids:
1. Acetic Acid (CH3COOH):
-
Acetic acid is used directly to enhance the flavour of food.
-
Vinegar which is used in food preparations and as preservatives is dilute solution of acetic acid.
-
It is also used as a cleansing agent in products meant for cleaning windows, floors, utensils, etc.
-
It also helps to remove stains on woodwork and carpets.
Use in food preservation:
|
FACT
-
Nitroglycerine has medical uses too.
-
It acts as a vasodilator (a chemical which widens blood vessels), so it is very useful in diseases related to circulation and the heart.
-
It is also anti-venom to the bite of the brown recluse spider.
|
-
Vinegar is used as preservative in common packaged food items such as pickles, sauces, ketchups, etc.
-
Most micro-organisms cannot survive in acidic environment.
-
An acidic environment either slows down their activities or can also kill them.



